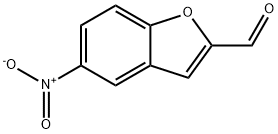
5-nitro-1-benzofuran-2-carbaldehyde synthesis
- Product Name:5-nitro-1-benzofuran-2-carbaldehyde
- CAS Number:23145-18-8
- Molecular formula:C9H5NO4
- Molecular Weight:191.14

90322-48-8
15 suppliers
$244.00/500mg
![4,1-Benzoxazepine-3-acetamide, 1-[3-(acetyloxy)-2,2-dimethylpropyl]-7-chloro-5-(2,3-dimethoxyphenyl)-1,2,3,5-tetrahydro-2-oxo-N-(propylsulfonyl)-, (3R,5S)-](/CAS/20210305/GIF/383653-04-1.gif)
383653-04-1
0 suppliers
inquiry

23145-18-8
13 suppliers
inquiry
Yield:23145-18-8 85%
Reaction Conditions:
with manganese dioxide in tetrahydrofuran;
Steps:
159.2 3-[5-[[[(3R,5S)-7-Chloro-5-(2,3-dimethoxyphenyl)-1-(3-hydroxy-2,2-dimethylpropyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]acetyl]amino]-1-benzofuran-2-yl]propanoic acid
(2) (5-Nitro-1-benzofuran-2-yl)methanol (0.19 g, 0.98 mmol) obtained in Example 159-(1) was dissolved in tetrahydrofuran (4 ml). Manganese dioxide (0.86 g, 9.84 mmol) was added at room temperature, and the mixture was stirred at 60° C. for 15 hours. The insolubles were filtered using Celite, the filtrate was concentrated under reduced pressure, the resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate=3:1), and dried under reduced pressure (50° C.) to obtain 2-formyl-5-nitro-1-benzofuran (0.16 g, yield 85.0%) as pale yellow crystals. 1H-NMR (200 MHz, CDCl3) δ: 7.72 (1H, s), 7.75 (1H, t, J=9.4 Hz), 8.45 (1H, d, J=9.4, 2.2 Hz), 8.74 (1H, d, J=2.2 Hz), 9.97 (1H, s). IR (KBr) 1696, 1524, 1350 cm-1. Elemental Analysis (C9H5NO4) Cal'd: C, 56.55; H, 2.64; N, 7.33. Found: C, 56.58; H, 2.82; N, 7.51.
References:
US2003/78251,2003,A1
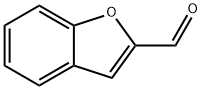
4265-16-1
191 suppliers
$24.00/1g

23145-18-8
13 suppliers
inquiry
50635-12-6
3 suppliers
inquiry

23145-18-8
13 suppliers
inquiry