
5-nitro-2,4-dimethoxypyrimidine synthesis
- Product Name:5-nitro-2,4-dimethoxypyrimidine
- CAS Number:30561-07-0
- Molecular formula:C6H7N3O4
- Molecular Weight:185.14
Yield:30561-07-0 88%
Reaction Conditions:
with sodium at 0 - 20;Inert atmosphere;
Steps:
Intermediate C-7A2: 2,4-dimethoxy-5-nitropyrimidine
To a 2 L three-neck round-bottom flask, containing thermometer, water condenser, connection to inert gas source (argon) and mechanical stirrer, was added methanol (1 L, HPLC grade) and under argon atmosphere small pieces of sodium (65 g, 2.83 mol, 2.2 eq) were slowly added for around 1 h with well stirring (reaction is exothermic, but the reaction mixture was not cooled; usually another sodium piece was added when first was consumed).
After dissolving the whole portion of sodium, reaction mixture was cooled to -5 °C and freshly prepared suspension of the C-7A1 (250 g, 1.29 mol, 1 eq) in methanol (500 mL, HPLC grade) was carefully added (around 40 minutes) in small portions with well stirring. (CAUTION: reaction is highly exothermic), one should avoid exceeding +10 °C during the addition.
Moreover, in the course of the reaction lots of solid product was formed.
After the whole amount of the substrate suspension was added, reaction mixture was maintained at 0 - 5 °C for around 0.5 h and then it was allowed to reach room temperature (usually it took around 2 h).
After that time, UPLCMS analysis showed complete consumption of the substrate.
Then, reaction mixture was cooled to around 5 °C and solid product was filtered, and washed with small amount (100 mL) of cold methanol.
The crude product was placed in a beaker with water (1 L) and it was well suspended with mechanic stirrer (around 10 minutes of well stirring).
The suspension was then filtered and obtained solid was washed with water (500 mL), n-hexane (200 mL) and dried on air overnight.
As a result, 211.5 g of the compound C-7A2 was obtained as a light yellow solid (88 % yield, 99 % purity according to UPLCMS analysis).
References:
EP3511334,2019,A1 Location in patent:Paragraph 0088-0089
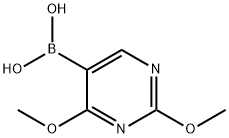
89641-18-9
190 suppliers
$20.00/1g

30561-07-0
37 suppliers
inquiry
49845-33-2
379 suppliers
$5.00/1g

30561-07-0
37 suppliers
inquiry

