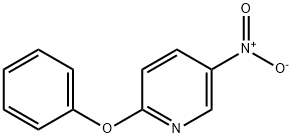
5-nitro-2-phenoxypyridine synthesis
- Product Name:5-nitro-2-phenoxypyridine
- CAS Number:28222-02-8
- Molecular formula:C11H8N2O3
- Molecular Weight:216.19
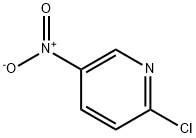
4548-45-2
538 suppliers
$6.00/5g

108-95-2
735 suppliers
$14.00/25g

28222-02-8
15 suppliers
$120.00/1g
Yield:28222-02-8 99%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 20; for 8 h;
Steps:
25
EXAMPLE 25 Preparation of 1H-indole-3-carboxylic acid(6-phenoxy-pyridine-3-yl)-amide Phenol (1.0 g, 10.6 mmol) was dissolved in DMF (20 ml), and the solution was stirred with NaH (430 mg, 10.51 mmol) and 2-chloro-5-nitro pyridine (1.68 g, 10.6 mmol) at room temperature for 8 hours. Subsequently, the product was filtered, washed, and dried under reduced pressures to yield a solid product (1.7 g, 99%). The solid product (2.0 g, 9.25 mmol) was added to a mixed solution of ethanol (20 ml) and conc. hydrochloric acid (5 ml), SnCl2 (5.26 g, 27.75 mmol) was added to the mixture, and the resulting solution was stirred at 50° C. for 3 hours. And then the product was filtered, washed, and dried to prepare 6-phenoxypyridine-3-amine (1.5 g, 87%).
References:
US2009/258876,2009,A1 Location in patent:Page/Page column 14
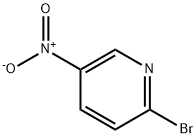
4487-59-6
438 suppliers
$5.00/1g

108-95-2
735 suppliers
$14.00/25g

28222-02-8
15 suppliers
$120.00/1g

4548-45-2
538 suppliers
$6.00/5g
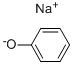
139-02-6
129 suppliers
$34.00/5g

28222-02-8
15 suppliers
$120.00/1g