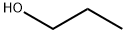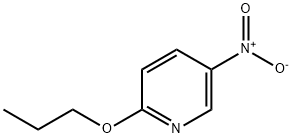
5-nitro-2-propoxy-pyridine synthesis
- Product Name:5-nitro-2-propoxy-pyridine
- CAS Number:99387-23-2
- Molecular formula:C8H10N2O3
- Molecular Weight:182
Yield:99387-23-2 0.480 g
Reaction Conditions:
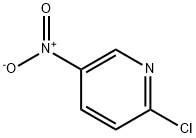
Stage #1: 2-chloro-5-nitropyridinewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;
Stage #2: propan-1-ol in N,N-dimethyl-formamide;mineral oil at 22 - 26;
Steps:
1 Step-i:- Preparation of 5-nitro-2-propoxypyridine
The title compound was prepared following the procedure described in step-3 of Intermediate-24 by using 2-chloro-5-nitropyridine (1.0 g, 6.32 mmol), propan-i-ol (0.760 g, 12.36 mmol), NaH (0.503 g, 12.57 mmol, 60% in mineral oil), DMF (5.0 mL) to afford 0.480 g title compound. ‘H NMR (300 MHz, DMSO-d6): 9.08 (s, 1H), 8.48-8.44 (dd, J = 7.2 Hz, 1H),7.04-7.01 (d, J = 9.3 Hz, 1H), 4.37-4.33 (t, J = 6.9 Hz, 2H), 1.79-1.72 (q, J = 6.9 Hz, 2H),0.99-0.94 (t, J= 7.8 Hz, 3H); MS [M+Hj: 182. :_To a solution of (5-(Qert-butyldimethylsilyl)oxy)-2-iodophenyl)methanol (i.0 g, 2.7i mmol) in dry DMF (iO mL) was added NaH (0.i64 g, 4.i2 mmol, 60% in mineral oil).The reaction mass was stirred for 30 mins at 0°C followed by addition of ethyl bromide (0.449 g, 4. i2 mmol). The reaction mixture was stirred at rt for 4-5 h.The reaction mixture was quenched with water and extracted with DCM. The organic layers were dried over Na2504 andconcentrated to afford 0.500 g of title compound. ‘H NMR (300 MHz, CDC13): 7.69-7.66 (d, J = 7.8Hz, iH), 6.83 (s, iH), 6.64-6.6i (d, J = 7.8Hz, iH), 4.68 (s, 2H), 4.i2-4.05 (q, J = 6.9Hz, 2H), i.49-i.45 (t, J= 6.9Hz, 3H), i.i4 (s, 9H), 0.i3 (s, 6H); MS [M+Hj: 363.
References:
WO2016/55947,2016,A1 Location in patent:Page/Page column 71; 103