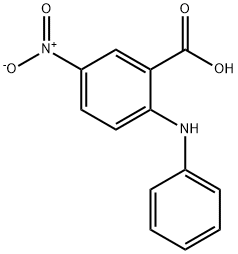
5-Nitro-N-phenylanthranilic acid synthesis
- Product Name:5-Nitro-N-phenylanthranilic acid
- CAS Number:16927-50-7
- Molecular formula:C13H10N2O4
- Molecular Weight:258.23
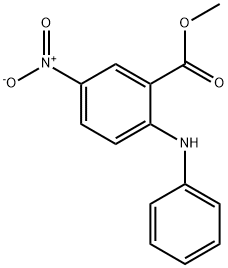
60042-00-4
0 suppliers
inquiry

16927-50-7
7 suppliers
$138.00/5mg
Yield:16927-50-7 90.5%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran;methanol;Reflux;
Steps:
General procedure: 3, 4, 5, 6: Hydrolysis of benzoic acidmethyl ester derivative(V, VI, and X) was accomplished by refluxing tetrahydrofuran/methanol (MeOH)/H2O (5:3:2) solution in the presence of lithiumhydroxide. The reaction mixture was acidified by the addition of1 M HCl(aq) then extracted with ethyl acetate and washed withbrine. The organic layer was dried over anhydrous MgSO4, filtered,and the solvent was evaporated to give anthranilic acid derivatives(3, 4, 5, and 6; Supplemental Methods, compound synthesis 1).
References:
Oh, Soo-Jin;Hwang, Seok Jin;Jung, Jonghoon;Yu, Kuai;Kim, Jeongyeon;Choi, Jung Yoon;Hartzell, H. Criss;Roh, Eun Joo;Justin Lee [Molecular Pharmacology,2013,vol. 84,# 5,p. 726 - 735] Location in patent:supporting information