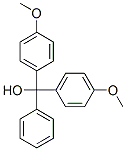
5'-O-Dimethoxytrityl-deoxythymidine synthesis
- Product Name:5'-O-Dimethoxytrityl-deoxythymidine
- CAS Number:40615-39-2
- Molecular formula:C31H32N2O7
- Molecular Weight:544.59
Yield:40615-39-2 96%
Reaction Conditions:
with pyridine at 20; for 12 h;
Steps:
5'-O-DMT-thymidine (7)
Thymidine (1.00 g; 4.13 mmol) was dissolved in pyridine (10 mL), DMT-Cl (1.90 g; 5.61 mmol; 1.36 equiv.) was added in portions. The reaction mixture was stirred at ambient temperature and the progress of the reaction was monitored by TLC. After complete consumption of the starting material (12 hours), MeOH (2 mL) was added and the resulting mixture was concentrated under vacuum. The residue was dissolved in CH2Cl2 and the organic phase was washed with satuarted aqueous NaHCO3 solution and dried over Na2SO4. After filtration the solvent was evaporated and the residue was purified by column chromatography (50 to 70% EtOAc in hexanes containing 1% triethylamine v/v). The product was then obtained as a yellow foam (2.1 g; 3.68 mmol; 96%). TLC Rf 0.36 (Hexanes/EtOAc 4:6; UV). 1H NMR (600 MHz, Acetonitrile-d3) δ 9.02 (s, 1H), 7.47-7.39 (m, 3H), 7.34-7.29 (m, 6H), 7.26-7.21 (m, 1H), 6.93-6.81 (m, 4H), 6.23 (t, J = 6.8 Hz, 1H), 4.49-4.41 (m, 1H), 3.92 (q, J = 3.6 Hz, 1H), 3.76 (s, 6H), 3.36 (d, J = 4.3 Hz, 1H), 3.30-3.24 (m, 2H), 2.30-2.20 (m, 2H), 1.48 (s, 3H). 13C NMR (151 MHz, Acetonitrile-d3) δ 164.60, 159.75, 151.41, 145.96, 136.85, 136.72, 136.68, 131.05, 131.03, 129.02, 128.91, 127.94, 114.11, 111.17, 87.38, 86.83, 85.24, 72.11, 64.62, 55.92, 40.80, 12.28. MS (ESI/TOF) m/z calculated for C31H32N2O7Na (M+Na)+ 567.2102 found 567.2004.
References:
Lingala, Suresh;Nordstr?m, Lars Ulrik;Mallikaratchy, Prabodhika R. [Tetrahedron Letters,2019,vol. 60,# 3,p. 211 - 213] Location in patent:supporting information

40615-36-9
540 suppliers
$5.00/1g

50-89-5
615 suppliers
$9.00/1g

40615-39-2
235 suppliers
$9.00/1g
![Thymidine, 3',5'-bis-O-[bis(4-methoxyphenyl)phenylmethyl]- (9CI)](/CAS/20211123/GIF/52417-09-1.gif)
52417-09-1
2 suppliers
inquiry
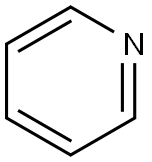
110-86-1
838 suppliers
$9.00/5g

40615-36-9
540 suppliers
$5.00/1g

50-89-5
615 suppliers
$9.00/1g
![Cytidine, N-benzoyl-5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxy-5-methyl-, 3'-benzoate (9CI)](/CAS/20210305/GIF/847699-97-2.gif)
847699-97-2
0 suppliers
inquiry

40615-39-2
235 suppliers
$9.00/1g
817623-11-3
12 suppliers
inquiry

40615-39-2
235 suppliers
$9.00/1g