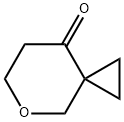
5-Oxaspiro[2.5]octan-8-one synthesis
- Product Name:5-Oxaspiro[2.5]octan-8-one
- CAS Number:1368764-75-3
- Molecular formula:C7H10O2
- Molecular Weight:126.15
![8,8-dimethoxy-5-oxaspiro[2.5]octane](/CAS/20180703/GIF/1421066-11-6.gif)
1421066-11-6
1 suppliers
inquiry
![5-Oxaspiro[2.5]octan-8-one](/CAS/20180703/GIF/1368764-75-3.gif)
1368764-75-3
11 suppliers
$1117.00/1g
Yield: 8%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20;
Steps:
215D synthesis of 5-Oxa-spiro[2.5]octan-8-one
Example 215C 8,8 -Dimethoxy- 5 -oxa- spiro [2.5 ] o ctane Mel2 (2 mL, 40 mmol) was added to a stirred solution of ZnEt2 (20 mL, 20 mmol) in dichloromethane (150 mL) at -78 °C under N2. The mixture was allowed to warm to 0 °C and stirred for 1 hour. A solution of Example 215B (1.58 g, 10 mmol) in dichloromethane (50 mL) was added, the resulting suspension was stirred at room temperature for 5 hours. Na2EDTA (100 mL) solution and NaHC03 (100 mL) solution were added to quench the reaction. The mixture was filtered, and the filtrate was extracted with EtOAc (200 mL). The combined organic layers were washed with brine, dried over Na2S04, filtered, and concentrated in vacuo. The residue was purified by preparative.TLC with 5 % ethyl acetate in hexanes to provide the title compound (200 mg, 12 %) as a yellow oil.
References:
ABBOTT LABORATORIES;ABBOTT LABORATORIES TRADING (SHANGHAI) COMPANY, LTD.;WANG, Xueqing;MEYER, Michael;YAO, Betty;GUO, Tao;WEI, Guo Ping,Robert;WANG, Lijuan, Jane WO2013/10453, 2013, A1 Location in patent:Page/Page column 266

693245-80-6
31 suppliers
$155.00/100mg
![5-Oxaspiro[2.5]octan-8-one](/CAS/20180703/GIF/1368764-75-3.gif)
1368764-75-3
11 suppliers
$1117.00/1g
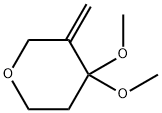
1421066-10-5
1 suppliers
inquiry
![5-Oxaspiro[2.5]octan-8-one](/CAS/20180703/GIF/1368764-75-3.gif)
1368764-75-3
11 suppliers
$1117.00/1g