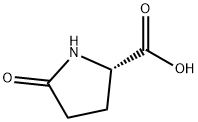
5-Oxo-1-phenyl-pyrrolidine-2-carboxylic acid synthesis
- Product Name:5-Oxo-1-phenyl-pyrrolidine-2-carboxylic acid
- CAS Number:18133-18-1
- Molecular formula:C11H11NO3
- Molecular Weight:205.21
Yield:18133-18-1 85%
Reaction Conditions:
Stage #1: Pyroglutamic acidwith 1,8-diazabicyclo[5.4.0]undec-7-ene in acetonitrile; for 0.5 h;
Stage #2: phenylboronic acidwith di-μ-hydroxo-bis[(N,N,N′,N′-tetramethylethylene-diamine)copper(II)] chloride in acetonitrile at 20; for 48 h;
Steps:
Synthesis of amide (±)-14
In a bulb of 10 mL, containing 4 mL of dry acetonitrile, 0.258 g of (±)-pyroglutamic acid ((±)-9) (2.00 mmol, 1 equiv) and 0.598 mL of 1,8-diazabicyclo[5.4.0]-undec-7-ene (4.00 mmol, 2 equiv) were brought together. After 10 minutes and 20 minutes, 0.139 g of di-μ-hydroxo-bis[(N,N,N′,N′-tetramethylethylene-diamine)copper(II)] chloride (0.300 mmol, 0.15 equiv) and 0.366 g of phenylboronic acid (13) (3.00 mmol, 1.5 equiv) were added, respectively, and the reaction mixture was stirred at room temperature for 48 hours. Afterwards, the solvent was evaporated in vacuo and the residue was dissolved in 30 mL of saturated aqueous NH4Cl and washed with ethyl acetate (3× 20 mL). The aqueous layer was acidified with 3-M aqueous HCl and extracted with ethyl acetate (3× 50 mL). The combined organic extracts were washed with brine (2× 25 mL) and dried over magnesium sulfate. Evaporation in vacuo afforded amide (±)-14 (0.349 g, 85%) as an off-white powder.
References:
Roman, Bart I.;Verhasselt, Sigrid;Mangodt, Christophe W.;De Wever, Olivier;Stevens, Christian V. [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 13,p. 2261 - 2264] Location in patent:supporting information
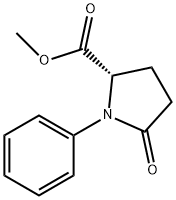
146500-35-8
10 suppliers
$279.23/1gm:

18133-18-1
32 suppliers
$45.00/10mg

108-86-1
493 suppliers
$10.00/5g

18133-18-1
32 suppliers
$45.00/10mg