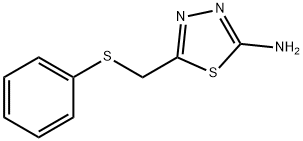
5-[(phenylthio)methyl]-1,3,4-thiadiazol-2-amine synthesis
- Product Name:5-[(phenylthio)methyl]-1,3,4-thiadiazol-2-amine
- CAS Number:88743-03-7
- Molecular formula:C9H9N3S2
- Molecular Weight:223.32

103-04-8
183 suppliers
$43.00/1g

79-19-6
503 suppliers
$14.00/1g
![5-[(phenylthio)methyl]-1,3,4-thiadiazol-2-amine](/CAS/GIF/88743-03-7.gif)
88743-03-7
10 suppliers
$126.00/500mg
Yield:88743-03-7 63%
Reaction Conditions:
Stage #1: 2-phenylthio-acetic acid;thiosemicarbazidewith trichlorophosphate at 75; for 0.5 h;
Stage #2: with lithium hydroxide monohydrate for 4 h;Reflux;
Stage #3: with sodium hydroxide in lithium hydroxide monohydrate at 20; pH=13 - 14;Reagent/catalyst;
Steps:
Synthesis of S-substituted 5-sulfanylmethyl(ethyl)-1,3,4-thiadiazol-2-amines (1a-k) (general procedure)
General procedure: Method .Sulfuric acid (98%, 24.2 mmol, 1.32 mL) was added to a mixture of the corresponding acid 2a-k (11 mmol) andthiosemicarbazide (0.91 g, 10 mmol). The resulting mixturewas heated either at 80-85 °C for 2-5 h (for 1a-c,i,j) or at 60-70 °C for 4-17 h (for 1d-h,k) and then cooled down to 20 °C. The reaction mixture was diluted with H2O (8 mL) and neutralized with aqueous NaOH solution (40%, 4 mL)upon cooling in an ice bath. The formed precipitate was fi lteredoff , washed with water, and dried in air.Method B. A mixture of the corresponding acid 2a-k(10 mmol), thiosemicarbazide (0.91 g, 10 mmol), and POCl3(2.6 ml, 28 mmol) was stirred at 75 °C for 0.5 h. The reactionmixture was cooled down to 20 °C, diluted with H2O (20 mL),and refluxed for 4 h. After cooling, the reaction mixture was treated with an aqueous NaOH solution (40%, ~17 mL, topH 13-14) and kept at 20 °C for 12-14 h. The precipitateformed was fi ltered off , washed with water, and dried in air. Compound 1e was recrystallized from ethanol. 5-[(Phenylsulfanyl)methyl]-1,3,4-thiadiazol-2-amine (1a).The yield was 58% (method , 4 h) or 63% (method B), whitepowder, m.p. 172-174 °C (cf. Ref. 26: 173-175 °C ). IR(KBr), /cm-1: 3255 (NH2), 1636, 1528, 1515 (C=N, C=C).1H NMR, : 4.42 (s, 2 H, CH2); 7.08 (s, 2 H, NH2); 7.20-7.24 (m, 1 H, Ph); 7.30-7.40 (m, 4 H, Ph). 13C NMR,: 31.4 (CH2); 126.4 (Ph); 128.8 (2 C, Ph); 129.0 (2 C,Ph); 134.4 (Ph); 156.2 (C=N); 169.0 (C=N). MS (ESI):found m/z 224.0312 [M + H]+; calculated for C9H10N3S2224.0311.
References:
Serkov;Sigai;Kostikova;Fedorov;Gazieva [Russian Chemical Bulletin,2022,vol. 71,# 8,p. 1801 - 1805][Izv. Akad. Nauk, Ser. Khim.,2022,# 8,p. 1801 - 1805]
5219-61-4
83 suppliers
$27.00/1g

79-19-6
503 suppliers
$14.00/1g
![5-[(phenylthio)methyl]-1,3,4-thiadiazol-2-amine](/CAS/GIF/88743-03-7.gif)
88743-03-7
10 suppliers
$126.00/500mg