
5-(PROP-1-EN-2-YL)PYRIDIN-2-AMINE synthesis
- Product Name:5-(PROP-1-EN-2-YL)PYRIDIN-2-AMINE
- CAS Number:848841-59-8
- Molecular formula:C8H10N2
- Molecular Weight:134.18
Yield:848841-59-8 880 mg
Reaction Conditions:
with potassium phosphate;(bis(tricyclohexyl)phosphine)palladium(II) dichloride in toluene; for 11 h;Reflux;Inert atmosphere;
Steps:
225 Reference Example 225
2-amino-5-(prop-1-en-2-yl)pyridine
A mixture of 2-amino-5-bromopyridine (1.00 g), 2-(propan-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.46 g), tripotassium phosphate (5.52 g) and dichlorobis(tricyclohexylphosphine)palladium (427 mg) in toluene (116 mL) was heated under reflux under a nitrogen atmosphere for 11 h
After cooling to room temperature, water was added, and the mixture was extracted twice with ethyl acetate.
The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated, and the obtained residue was purified by silica gel column chromatography (solvent; hexane/ethyl acetate=70/30 - 0/100) to give the title compound (880 mg).
MS(ESI)m/z; 135[M+H]+
References:
EP3372601,2018,A1 Location in patent:Paragraph 0588; 0589

1072-97-5
692 suppliers
$9.00/1g
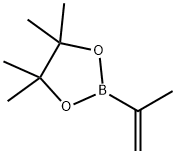
126726-62-3
178 suppliers
$38.50/1g

848841-59-8
6 suppliers
inquiry
848841-58-7
6 suppliers
inquiry

848841-59-8
6 suppliers
inquiry
