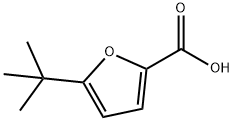
5-tert-butyl-2-furoic acid synthesis
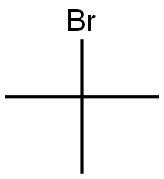
- Product Name:5-tert-butyl-2-furoic acid
- CAS Number:56311-39-8
- Molecular formula:C9H12O3
- Molecular Weight:168.19

7040-43-9
19 suppliers
$138.00/1g
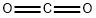
124-38-9
124 suppliers
$175.00/23402

56311-39-8
24 suppliers
inquiry
Yield: 69%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane
Steps:
K.1 Step 1
Step 1 To a solution of 2-tert-butylfuran (4.5 g, 36 mmol) in anh. THF (60 mL) at -78° C. under N2 was added n-butyllithium (1.6M in hexane, 25 mL, 40 mmol, 1.1 equiv) dropwise. After 30 min, the cooling bath was replaced with an ice bath and the mixture was stirred at 0° C. for 1 h. Dry CO2, generated from dry ice and dried over an anhydrous Na2SO4 tower, was bubbled into the reaction mixture over 20 min at -78° C. and then at 0° C. The reaction mixture was acidified to pH 1 with a 1M HCl solution, then extracted with EtOAc. The organic layer was washed with a concentrated NaCl solution, dried (NaSO4) and concentrated under reduced pressure to give 5-tert-butylfuran-2-carboxylic acid as a pale yellow solid (4.2 g, 69%): 1H NMR (CDCl3) δ1.29 (s, 9H), 6.11 (d 1H, J=3.3 Hz), 7.19 (d, 1H, J=3.3 Hz), 11.0 (br s, 1H).
References:
Bayer Corporation US6344476, 2002, B1
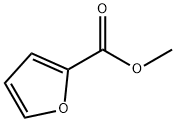
611-13-2
406 suppliers
$13.00/25g
507-20-0
361 suppliers
$13.00/25mL

56311-39-8
24 suppliers
inquiry

611-13-2
406 suppliers
$13.00/25g

109-69-3
421 suppliers
$19.00/25mL

56311-39-8
24 suppliers
inquiry