
5-Thiazolemethanol, 2-bromo-α-(trifluoromethyl)- synthesis
- Product Name:5-Thiazolemethanol, 2-bromo-α-(trifluoromethyl)-
- CAS Number:929028-45-5
- Molecular formula:C5H3BrF3NOS
- Molecular Weight:262.05
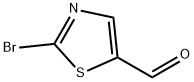
464192-28-7
156 suppliers
$12.00/250mg
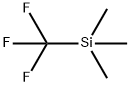
81290-20-2
408 suppliers
$13.00/1g

929028-45-5
1 suppliers
inquiry
Yield:929028-45-5 48%
Reaction Conditions:
with cesium fluoride in 1,2-dimethoxyethane at 20; for 2 h;
Steps:
5; 149.1
A solution (0.52 M) of 2-bromo-5-formylthiazole and CsF (0.2 eq.) in DME was treated with TMSCF3(2.0 eq.) and the resulting solution was stirred at RT for 2 h. Then, the reaction mixture was quenched with water and stirred at RT for 15 min. The aqueous solution was extracted with EtOAc several times and the combined organic layers were dried and concentrated to give a residue that was purified by flash chromatography on silica gel (petroleum ether/EtOAc 20: 1 to 3: 1) to yield (48%) the title compound as a yellow solid.1H NMR (300 MHz, CDCl3, 300K) δ 4.04 (d, J= 5.9 Hz, IH), 5.32 (m, IH), 7.59 (s, IH); MS (ES+)C5H3BrF3NOS requires: 261, 263, found: 262, 264 (M+H)+.
References:
WO2007/29035,2007,A2 Location in patent:Page/Page column 57; 73-74