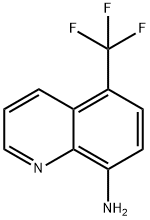
5-trifluoroMethyl-8-quinolinaMine synthesis
- Product Name:5-trifluoroMethyl-8-quinolinaMine
- CAS Number:483-69-2
- Molecular formula:C10H7F3N2
- Molecular Weight:212.17

917251-92-4
37 suppliers
$44.00/100mg

483-69-2
32 suppliers
$143.00/100mg
Yield:483-69-2 80 %
Reaction Conditions:
with Benzophenone imine;palladium diacetate;caesium carbonate;Xantphos in toluene at 100;
Steps:
47 5-(Trifluoromethyl)qiiinolin-8-amine.
To a stirred degassed solution of 8-bromo-5-(trifluoromethyl)quinoline (500 mg, 1.82 mmol) in toluene (8 ml) was added benzophenone imine (395 mg, 2.18 mmol) followed by CS2CO3 (289 mg, 2.72 mmol), Pd(OAc)2 (41 mg, 0.18 mmol) and xantphos (210 mg, 0.36 mmol). Resulting mixture was heated at 100 °C for 16h. Reaction mixture was cooled to ambient temperature, filtered through a short pad of celite and washed with DCM. Solvent was removed under reduced pressure and the residue was dissolved in EtOH-THF (10 mL, 1:1) and acidified to pH 1 with 1 M aqueous HCI solution. Resulting mixture was stirred at room temperature for 3 h. It was then basified with saturated aqueous NaHCCh solution and extracted with ethyl acetate. Combined organic layer was washed with brine and dried over anhydrous NaiSOr, fdtered and concentrated under reduced pressure. Crude product was purified by combiflash chromatography (20-25% EtOAc in hexane) to afford 5-(trifluoromethyl)quinolin-8-amine (310 mg, 80%) as orange solid. MS (ESI): m/z 200.3 [M+l]+.
References:
WO2022/173727,2022,A1 Location in patent:Paragraph 00396
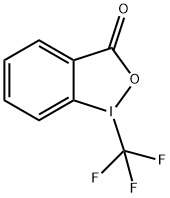
887144-94-7
176 suppliers
$19.00/1g
578-66-5
324 suppliers
$6.00/1g
313-16-6
8 suppliers
inquiry

483-69-2
32 suppliers
$143.00/100mg

578-66-5
324 suppliers
$6.00/1g

483-69-2
32 suppliers
$143.00/100mg
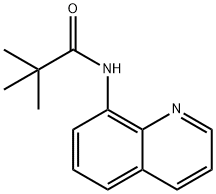
170891-76-6
1 suppliers
inquiry

483-69-2
32 suppliers
$143.00/100mg
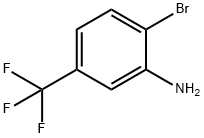
454-79-5
231 suppliers
$22.00/1g

483-69-2
32 suppliers
$143.00/100mg