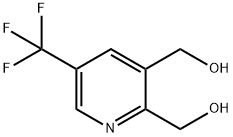
(5-(TRIFLUOROMETHYL)PYRIDINE-2,3-DIYL)DIMETHANOL synthesis
- Product Name:(5-(TRIFLUOROMETHYL)PYRIDINE-2,3-DIYL)DIMETHANOL
- CAS Number:905273-63-4
- Molecular formula:C8H8F3NO2
- Molecular Weight:207.15
120083-60-5
16 suppliers
$45.00/10mg

905273-63-4
5 suppliers
inquiry
Yield:905273-63-4 76.9%
Reaction Conditions:
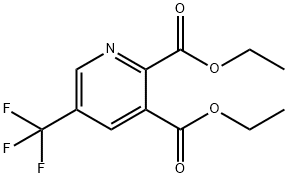
Stage #1: diethyl 5-(trifluoromethyl)pyridine-2,3-dicarboxylatewith sodium tetrahydroborate;ethanol;magnesium chloride in tetrahydrofuran at 10 - 32; for 14.5 - 15 h;
Stage #2: with water;sodium hydrogencarbonate in tetrahydrofuran;ethanol; for 0.6 h;
Steps:
2.1
A suspension of MgCl2 (1.63 kg, 16.8 mol) in THF (14.5 L) was treated at Timax=10° C. over 35 min with EtOH (11.8 L) (exothermic reaction). The reaction mixture was vigorously stirred and treated in portions (over 30-60 min, temperature controlled) with a suspension of sodium borohydride (1.3 kg, 32.99 mol) in THF (6.7 L). A very exothermic reaction ensued, accompanied by foaming. The mixture was warmed to 28° C. and stirred at this temperature over 20 min. To the suspension was added over 35 min at Ti=27-32° C. (Ti=internal temperature) a solution of 2,3-pyridine dicarboxylic acid-5-trifluoromethyl diethyl ester (1.69 kg, 5.7 mol) in EtOH (5.1 L). The yellow-orange suspension was stirred for 13 h at Ti=27-32° C.The yellow reaction mixture was added in portions over 18 min to a solution of NaHCO3 (5.9 kg, 81.9 mol) and H2O (68 L) resulting in an exothermic reaction and gas evolution. The yellow suspension was stirred for 18 min, then all of the organic solvent was removed by evaporation under reduced pressure (400-50 mbar) at 60° C. The total volume was adjusted with water to 70 L. The suspension was treated under vigorous stirring with citric acid (10.5 kg, 54.3 mol) dissolved in H2O (8.4 L) to adjust the pH to 7 (foaming). To the yellow solution was added 40 l tert-butyl methyl ether. After extraction and phase separation, the water phase was extracted twice with tert-butyl methyl ether (30 L, 60 Lin total). The combined organic phases were dried (15 kg Na2SO4), filtered and evaporated at 60° C. and 750-10 mbar to afford 913.0 g (76.9%) of (2-hydroxymethyl-5-trifluoromethyl-pyridin-3-yl)-methanol as a yellow-red oil: MS (EI): 208 (M+H+, 13), 289 (54), 161 (100).
References:
US2009/143589,2009,A1 Location in patent:Page/Page column 5
![Furo[3,4-b]pyridin-7(5H)-one, 3-(trifluoromethyl)-](/CAS/20210305/GIF/765298-32-6.gif)
765298-32-6
0 suppliers
inquiry

905273-63-4
5 suppliers
inquiry