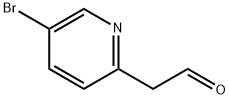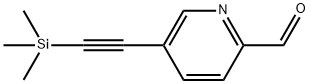
5-((trimethylsilyl)ethynyl)picolinaldehyde synthesis
- Product Name:5-((trimethylsilyl)ethynyl)picolinaldehyde
- CAS Number:650606-63-6
- Molecular formula:C11H13NOSi
- Molecular Weight:203.31
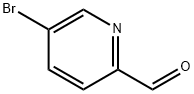
31181-90-5
332 suppliers
$5.00/250mg
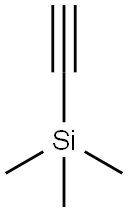
1066-54-2
470 suppliers
$9.00/10g

650606-63-6
8 suppliers
inquiry
Yield:650606-63-6 95%
Reaction Conditions:
with copper (I) iodide;triethylamine;[1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II);triphenylphosphine in tetrahydrofuran at 100; for 0.166667 h;Inert atmosphere;Microwave irradiation;
Steps:
A62.A
Example A62. (lR^SVfEVS'-methoxy^-G-CfEl^-f-fpiDeridin-l-ylmethvnpyridin-S- yl)vinγI)-l H-indazol-6-yl)spiro[cyclopropane-l,3'-indolinl-2'-one 2,2,2-trifluoroacetate saltA. 5-((trimethylsilyl)ethynyl)picolinaldehyde A solution of 5-bromopicolinaldehyde (1 g, 5.3 mmol), TMS-acetylene (1.04 g, 10.6 mmol) and triethylamine (4 mL) in THF (10 mL) was purged with argon for 10 min. Copper iodide (76 mg, 0.4 mmol), Pd(PPh3)2Cl2 (141 mg, 0.4 mmol) and triphenylphosphine (0.1 g, 0.4 mmol) was added and the mixture was heated to 1000C 5 under microwave irradiation for 10 min. Ethyl acetate (200 mL) was added at the solution was washed with water (2 x 25 mL), dried over MgSO4 and concentrated to dryness. The residue was filtered through silica gel with CH2Cl2 to give the title compound as a yellow oil (1 g, 95%). 1H NMR (400 MHz, CDCl3) δ 10.08 (s, I H), 8.82 (s, I H), 7.91 (s, 2H), 0.29 (s, 9H). Q
References:
WO2010/115279,2010,A1 Location in patent:Page/Page column 180-181
624-28-2
713 suppliers
$5.00/5g

650606-63-6
8 suppliers
inquiry