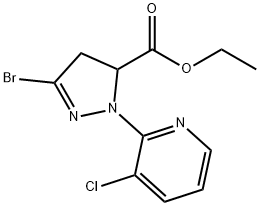
ETHYL 1-(3-CHLORO-2-PYRIDINYL)-3-PYRAZOLIDINONE-5-CARBOXYLATE synthesis
- Product Name:ETHYL 1-(3-CHLORO-2-PYRIDINYL)-3-PYRAZOLIDINONE-5-CARBOXYLATE
- CAS Number:500011-88-1
- Molecular formula:C11H12ClN3O3
- Molecular Weight:269.68
500011-91-6
14 suppliers
inquiry

500011-88-1
31 suppliers
inquiry
Yield:500011-88-1 90%
Reaction Conditions:
Stage #1: 5-bromo-2-(3-chloro-pyridin-2-yl)-3,4-dihydro-2H-pyrazole-3-carboxylic acid ethyl esterwith sulfuric acid in acetonitrile at 22 - 36;
Stage #2: with potassium peroxodisulfate at 84; for 2 h;
Steps:
1.C Step C: Preparation of Ethyl [3-BROMO-1-(3-CHLORO-2-PYRIDINYL)-LH-PYRAZOLE-5-] carboxylate
A [1-L] four-necked flask equipped with a mechanical stirrer, thermometer, reflux condenser, and nitrogen inlet was charged with ethyl [3-BROMO-L- (3-CHLORO-2-PYRIDINYL)-] 4, 5-dihydro-lH-pyrazole-5-carboxylate (i. e. the product of Step B) (40.2 g, 0.121 mol), acetonitrile (300 mL) and sulfuric acid (98%, 13.0 [ML,] 0.245 mol). The mixture self-heated from 22 to [36 °C] upon adding the sulfuric acid. After being stirred for several minutes, the mixture was treated with potassium persulfate (48.0 g, 0.178 mol). The slurry was heated to reflux at [84 °C] for 2 hours. The resulting orange slurry while still warm [(50-65 °C)] was filtered to remove a white precipitate. The filter cake was washed with acetonitrile (2 x 50 mL). The filtrate was concentrated to about 200 [ML] on a rotary evaporator. A second 1-L four-necked flask equipped with a mechanical stirrer was charged with water (400 mL). The concentrated reaction mass was added to the water over a period of about 5 minutes. The product was isolated via filtration, washed sequentially with aqueous acetonitrile (20%, 100 mL) and water (75 mL), and was then air-dried on the filter for 1 hour. The product consisted of a crystalline, orange powder (36.6 g, 90% yield). The only appreciable impurities observed by 1H NMR were about 1% of an unknown and 0.5% acetonitrile. 1H NMR (DMSO-d6) 8 1.09 (t, 3H), 4.16 (q, 2H), 7.35 (s, 1H), 7.72 (dd, 1H), 8.39 (d, 1H), 8.59 (d, 1H).
References:
WO2004/33468,2004,A1 Location in patent:Page 19

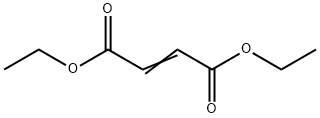
64-17-5
706 suppliers
$10.00/50g
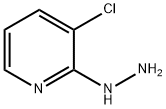
22841-92-5
215 suppliers
inquiry

141-05-9
447 suppliers
$6.00/25g

500011-88-1
31 suppliers
inquiry