5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo- synthesis
- Product Name:5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo-
- CAS Number:501364-82-5
- Molecular formula:C23H25N3O4S
- Molecular Weight:439.53
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo-](/CAS/GIF/501364-82-5.gif)
501364-82-5
11 suppliers
inquiry
Yield: 44%
Reaction Conditions:
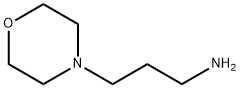
Stage #1:4-(3-Aminopropyl)morpholine with sodium hydrogencarbonate in water;ethyl acetate for 0.25 h;
Stage #2:9-chlorosulfonyl-5,6-dihydro-5-oxo-indeno[1,2-c]isoquinoline in water;ethyl acetate at 20; for 24 h;Product distribution / selectivity;
Steps:
1.7; 6.1.h.I
To a stirred suspension of 3-(4-morpholino)-1-propylamine (17. 28 g, 0.12 mol) in EtOAc was added sat. aq. NaHCO3 (300 mL), and the mixture was allowed to stir for 15 min. Compound 7 (4.0 g, 0.012 mol) was then introduced in small portions at room temperature. The reaction mixture was stirred for 24 h; filtered and washed with H2O, EtOAc and MeOH ; refluxed in MeOH for 30 min ; filtered while still warm; and washed with MeOH to provide compound 81 as a free base (2.33 g, 44 %).'H NMR (DMSO-d6): 81. 47-1.52 (m, 2H), 2.16-2. 21 (m, 4H), 2.47-2. 48 (m, 2H), 3.44-3. 48 (m, 2H), 3.23 (m, 4H), 4.02 (s, 2H), 7.49-7. 58 (m, lH), 7.78-7. 82 (m, 3H), 7.97 (s, 1H), 8.14 (d, J= 7. 8 Hz, 1H), 8.26 (d, J = 7.8 Hz, 1H), 9.59 (s, 1H), 12.42 (s, 1H).
References:
INOTEK PHARMACEUTICALS CORPORATION WO2005/82368, 2005, A1 Location in patent:Page/Page column 60; 86-87
![5H-indeno[1,2-c]isoquinoline-5,11(6H)-dione](/CAS/20200401/GIF/5264-21-1.gif)
5264-21-1
1 suppliers
inquiry
![5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo-](/CAS/GIF/501364-82-5.gif)
501364-82-5
11 suppliers
inquiry

5651-60-5
9 suppliers
inquiry
![5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo-](/CAS/GIF/501364-82-5.gif)
501364-82-5
11 suppliers
inquiry
![6,11-dihydro-5H-indeno[1,2-c]isoquinolin-5-one](/CAS/20200331/GIF/109722-74-9.gif)
109722-74-9
1 suppliers
inquiry
![5H-Indeno[1,2-c]isoquinoline-9-sulfonaMide, 6,11-dihydro-N-[3-(4-Morpholinyl)propyl]-5-oxo-](/CAS/GIF/501364-82-5.gif)
501364-82-5
11 suppliers
inquiry