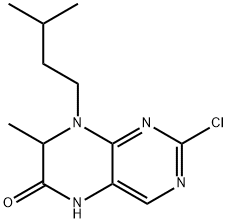
2-Chloro-8-isopentyl-7-methyl-7,8-dihydropteridin-6(5H)-one synthesis
- Product Name:2-Chloro-8-isopentyl-7-methyl-7,8-dihydropteridin-6(5H)-one
- CAS Number:501439-14-1
- Molecular formula:C12H17ClN4O
- Molecular Weight:268.74
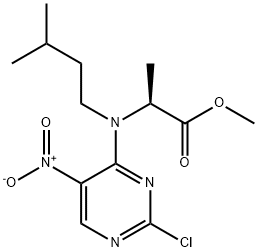
1435462-61-5
0 suppliers
inquiry

501439-14-1
31 suppliers
inquiry
Yield:501439-14-1 61%
Reaction Conditions:
Stage #1: methyl N-(2-chloro-5-nitropyrimidin-4-yl)-N-(3-methylbutyl)alaninatewith iron;acetic acid at 60; for 2 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
1.c Step c: 2-Chloro-7-methyl-8-(3-methylbutyl)-7,8-dihydropteridin-6(5/-/)-one
A mixture of methyl /V-(2-chloro-5-nitropyrimidin-4-yl)-/V-(3-methylbutyl)alaninate (16.2 g, 49 mmol), iron powder (6.9 g, 123 mmol) and glacial acetic acid (165 mL) was heated at 60°C for 2 h. The reaction mixture was then filtered while hot over celite and washed with hot acetic acid (40 mL) followed by ethyl acetate (250 mL). The filtrate after evaporation was taken up in ethyl acetate and washed two times with aq. saturated sodium bicarbonate solution followed by brine. It was dried over sodium sulfate and evaporated. The residue was triturated with hot isopropyl ether to give titled product (yield: 61 %). 1HNMR (300MHz, CDCI3): δ 0.92-1 .01 (m, 7H), 1.43-1.75 (m, 5H), 3.01 -3.20 (m, 1 H), 4.10-4.25 (m, 2H), 7.63 (s, 1 H), 9.13 (s, 1 H).
References:
WO2013/71217,2013,A1 Location in patent:Page/Page column 30-32
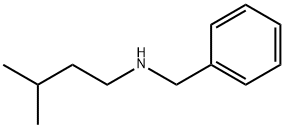
4462-17-3
7 suppliers
inquiry

501439-14-1
31 suppliers
inquiry
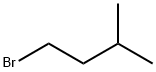
107-82-4
277 suppliers
$13.00/25g

501439-14-1
31 suppliers
inquiry
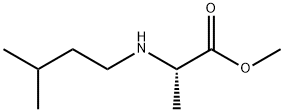
1435462-60-4
1 suppliers
inquiry

501439-14-1
31 suppliers
inquiry

590-86-3
368 suppliers
$10.00/5g

501439-14-1
31 suppliers
inquiry