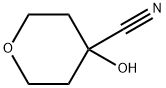
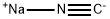2H-Pyran-4-carboxylicacid,tetrahydro-4-hydroxy-(9CI) synthesis
- Product Name:2H-Pyran-4-carboxylicacid,tetrahydro-4-hydroxy-(9CI)
- CAS Number:50289-13-9
- Molecular formula:C6H10O4
- Molecular Weight:146.14
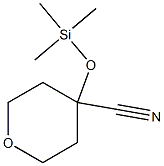
112799-02-7
4 suppliers
inquiry

50289-13-9
31 suppliers
inquiry
Yield:50289-13-9 15.05 g
Reaction Conditions:
with hydrogenchloride;glacial acetic acid at 0 - 90; for 4 h;
Steps:
5.2 [0215] Step 2: 4-hydroxyoxane-4-carboxylic acid (32)
[0216] A stirred mixture of 4-[(trimethylsilyl)oxy]oxane-4-carbonitrile 31 (20.69 g, 103.80 mmol) and glacial AcOH (45 mL) was cooled to 0 °C. Concentrated HCl (37%, 45 mL) was added dropwise at 0 °C, the cooling bath was then removed, and the resulting mixture was heated at 90 °C for 4 h. Subsequently, the reaction mixture was cooled to room temperature and concentrated under reduced pressure. The residue was diluted with water (80 mL) and the aqueous phase was extracted with ethyl acetate (4 X 120 mL). The aqueous layer was then saturated with solid NaCl and further extracted with ethyl acetate (4 X 120 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo. Trace amounts of solvents were removed by co-evaporation with toluene to afford 4-hydroxyoxane-4-carboxylic acid 32 (15.05 g, 99%) as a light brown solid.[0217] 1H NMR (400 MHz, DMSO-d6) δ 5.26 (br s, 1H), 3.68 - 3.56 (m, 4H), 1.96 - 1.79 (m, 2H), 1.55 - 1.42 (m, 2H). [0218] MS (ESI) m/z: [M-1]-145.0
References:
WO2022/208356,2022,A1 Location in patent:Paragraph 0215-0218
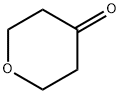
29943-42-8
425 suppliers
$5.00/1g

50289-13-9
31 suppliers
inquiry
50289-10-6
17 suppliers
inquiry

50289-13-9
31 suppliers
inquiry