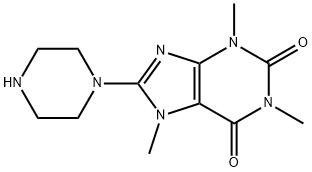
1,3,7-TRIMETHYL-8-PIPERAZIN-1-YL-3,7-DIHYDRO-PURINE-2,6-DIONE synthesis
- Product Name:1,3,7-TRIMETHYL-8-PIPERAZIN-1-YL-3,7-DIHYDRO-PURINE-2,6-DIONE
- CAS Number:50693-74-8
- Molecular formula:C12H18N6O2
- Molecular Weight:278.31
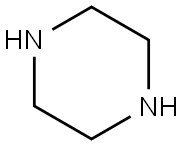
110-85-0
563 suppliers
$5.00/5G

4921-49-7
32 suppliers
$172.00/50MG

50693-74-8
19 suppliers
$300.00/1g
Yield:50693-74-8 90%
Reaction Conditions:
in 2-methoxy-ethanol at 120; for 3 h;Microwave irradiation;
Steps:
Synthesis of 8-Amino-substituted Caffeine Derivatives. General Method.
General procedure: 8-Chlorocaffeine (5, 0.44 mmol,100 mg) and the appropriate amine [ethylenediamine (8), hexamethylenediamine (9), piperazine (11), or morpholine (12)](1.76 mmol) were dissolved in methoxyethanol (3.5 mL). The mixture was stirred for 3 h in a microwave reactor at 120°C andcooled. The resulting precipitate of disubstitution product (0.5-4% yield) was filtered off and rinsed with EtOH (3 × 5 mL).The mother liquor was evaporated. The residue was worked up with H2O and extracted with CHCl3 (3 × 20 mL). The organicfractions were combined, dried over MgSO4, and evaporated. Compounds 6, 7, 13, and 14 were purified by columnchromatography over silica gel (CHCl3-EtOH eluent, 100:0→70:30).
References:
Reshetnikov;Patrushev;Shults [Chemistry of Natural Compounds,2020,vol. 56,# 5,p. 855 - 860][Khim. Prir. Soedin.,2020,vol. 5,p. 733 - 737,5]

110-85-0
563 suppliers
$5.00/5G
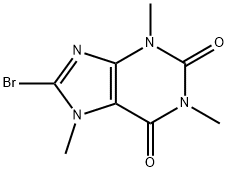
10381-82-5
19 suppliers
$78.79/1G

50693-74-8
19 suppliers
$300.00/1g
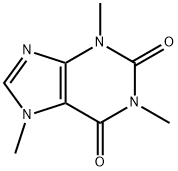
58-08-2
10 suppliers
$12.00/1mg

50693-74-8
19 suppliers
$300.00/1g