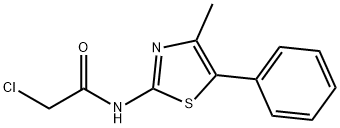
Acetamide, 2-chloro-N-(4-methyl-5-phenyl-2-thiazolyl)- synthesis
- Product Name:Acetamide, 2-chloro-N-(4-methyl-5-phenyl-2-thiazolyl)-
- CAS Number:50772-55-9
- Molecular formula:C12H11ClN2OS
- Molecular Weight:266.75
Yield:-
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;
Steps:
2-Chloro-N-(5-substituted-4-methylthiazole-2-yl)acetamideDerivatives (2)
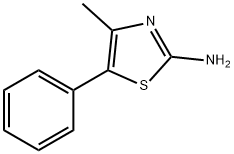
General procedure: A solution of 2-amino-5-substituted-4-methylthiazole (1) (0.01 mol) (1a:1.2 g, 1b:1.3 g, 1c:1.6 g, 1d:1.9 g, 1e:1.9 g)[29, 30] and triethylamine (0.01 mol) (1.5 mL) was prepared by stirring in THF (50 mL) at room temperature. After cooling the mixture in an ice bath, chloroacetyl chloride (0.01mol) (0.8 mL) was added drop wise with constant stirring. Before evaporation of the solvent under reduced pressure,the reaction mixture was further stirred for 1 hour at room temperature. The precipitate formed was crystallised from ethanol [31, 12].
References:
Gundogdu-Karaburun, Nalan [Letters in drug design and discovery,2014,vol. 11,# 6,p. 814 - 823]