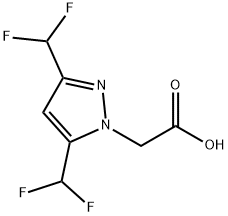
[3,5-bis(difluoromethyl)-1H-pyrazol-1-yl]acetic acid synthesis
- Product Name:[3,5-bis(difluoromethyl)-1H-pyrazol-1-yl]acetic acid
- CAS Number:512809-60-8
- Molecular formula:C7H6F4N2O2
- Molecular Weight:226.13
1006482-19-4
0 suppliers
inquiry
![[3,5-bis(difluoromethyl)-1H-pyrazol-1-yl]acetic acid](/CAS/GIF/512809-60-8.gif)
512809-60-8
12 suppliers
$60.00/50mg
Yield:512809-60-8 87%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran;methanol at 0 - 27; for 16 h;Inert atmosphere;
Steps:
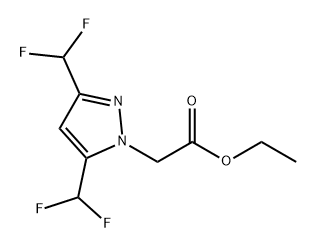
Preparation of 2-(3,5-bis(difluoromethyl)-1H-pyrazol-1-yl)acetic
To a stirred solution of ethyl 2-(3,5-bis(difluoromethyl)-1H-pyrazol-1-yl)acetate (22 g, 75 mmol) in THF (50 mL) and methanol (25 mL) under N2 atmosphere at 0°C was added dropwise a solution of lithium hydroxide (5.41 g, 226 mmol) in water (25 mL). The reaction mixture was allowed to warm to 27 °C and was then stirred for 16 h at that temperature. Progress of the reaction was monitored by TLC (SiO2, 50% EtOAc/pet, Rf = 0.2, UV-active). After completion, the reaction mixture was concentrated under reduced pressure. The resulting residue was dissolved in water (100 mL) and the solution was adjusted to pH 3 using aq. HCl (2 N). The solution was extracted with EtOAc (4 X 50 mL). The combined organic layers were washed with brine (50 mL), dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure to afford 2-(3,5-bis(difluoromethyl)-1H-pyrazol-1-yl)acetic as pale-yellow solid acid, 15 g (87 %).1H NMR (400 MHz, DMSO-d6) d = 13.53 -13.24 (m, 1H), 7.46 -7.07 (m, 3H), 5.14 (s, 2H ). LCMS: (M-H) = 225.15; LCMS Purity = 98.7%.
References:
WO2020/84492,2020,A1 Location in patent:Page/Page column 46-47

70086-62-3
55 suppliers
$19.00/1g
![[3,5-bis(difluoromethyl)-1H-pyrazol-1-yl]acetic acid](/CAS/GIF/512809-60-8.gif)
512809-60-8
12 suppliers
$60.00/50mg