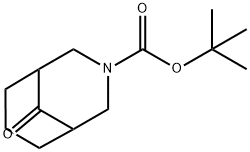
tert-butyl 9-oxo-3-azabicyclo[3.3.1]nonane-3-carboxylate synthesis
- Product Name:tert-butyl 9-oxo-3-azabicyclo[3.3.1]nonane-3-carboxylate
- CAS Number:512822-34-3
- Molecular formula:C13H21NO3
- Molecular Weight:239.31

24424-99-5
840 suppliers
$13.50/25G
![3-azabicyclo[3.3.1]nonan-9-one](/CAS/20150408/GIF/86949-84-0.gif)
86949-84-0
9 suppliers
inquiry
![tert-butyl 9-oxo-3-azabicyclo[3.3.1]nonane-3-carboxylate](/CAS/20150408/GIF/512822-34-3.gif)
512822-34-3
35 suppliers
$208.33/0.25g
Yield:512822-34-3 0.3 g
Reaction Conditions:
with diisopropylamine in dichloromethane at 20; for 1 h;
Steps:
Step 2.
To a crude solution of 3-azabicyclo[3.3.1]nonan-9-one (0.35 g, 1.0 eq., 2.51 mmol) in dry DCM (5.0 mL) were sequentially added di-tert-butyl dicarbonate (0.656 g, 1.2 eq., 3.01 mmol) and diisopropyl amine (1.31 mL, 3.0 eq., 7.53 mmol). The resulting mixture was stirred at room temperature for 1 h. The reaction was then quenched with water and the organics extracted with DCM (220 mL). The organic phase was dried over Na2SO4 and concentrated in vacuo to furnish a crude product, which was purified by flash chromatography eluting with cyclohexane/EtOAc (8:2) to give the pure tert-butyl 9-oxo-3-azabicyclo[3.3.1]nonane-3-carboxylate as a pale yellow solid (0.3 g, 50% estimated). UPLC-MS: tR=2.23 min (generic method); MS (ESI) m/z calcd for C9H14NO3 (M-tBu+2H)+: 184.1, found: 184.1. 1H NMR (400 MHz, DMSO-d6) 4.43-4.18 (m, 2H), 3.35-3.10 (m, 2H), 2.27 (br s, 2H), 2.17-2.03 (m, 2H), 1.99-1.82 (m, 3H), 1.49-1.45 (m, 1H), 1.44 (s, 9H).
References:
US2019/135778,2019,A1 Location in patent:Paragraph 0107
![9-BENZYL-9-AZABICYCLO[3.3.1]NONAN-3-ONE](/CAS/GIF/81879-64-3.gif)
81879-64-3
49 suppliers
$61.00/100mg
![tert-butyl 9-oxo-3-azabicyclo[3.3.1]nonane-3-carboxylate](/CAS/20150408/GIF/512822-34-3.gif)
512822-34-3
35 suppliers
$208.33/0.25g