
5-(aminomethyl)furan-2-carboxylic acid hydrochloride synthesis
- Product Name:5-(aminomethyl)furan-2-carboxylic acid hydrochloride
- CAS Number:51521-95-0
- Molecular formula:C6H8ClNO3
- Molecular Weight:177.5856
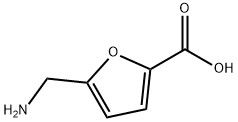
934-65-6
26 suppliers
$72.00/100mg

51521-95-0
17 suppliers
inquiry
Yield:51521-95-0 100%
Reaction Conditions:
with hydrogenchloride in water;
Steps:
2.1 4.2.1 5-(Aminomethyl)furan-2-carboxylic acid hydrochloride (4a)
5-(Aminomethyl)furan-2-carboxylic acid (25.0 mg, 0.18 mmol) was dissolved in 2 N HCl (5 mL) followed by solvent removal under reduced pressure. This was repeated twice to yield 5-(aminomethyl)furan-2-carboxylic acid hydrochloride as an off white powder (31.5 mg, 100%). 1H NMR (500 MHz, MeOD) δ 7.22 (d, J = 3.3 Hz, 1H), 6.71 (d, J = 3.3 Hz, 1H), 4.25 (s, 2H). 13C NMR (126 MHz, MeOD) δ 161.27, 152.05, 147.49, 119.76, 113.69, 36.80. HRMS (ESI): Calcd for C6H7NO3 [M-H]- 140.0353; found 140.0343.
References:
Hawker, Dustin D.;Silverman, Richard B. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 19,p. 5763 - 5773]
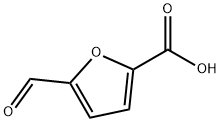
13529-17-4
149 suppliers
$16.00/100mg

51521-95-0
17 suppliers
inquiry

2528-00-9
118 suppliers
$12.00/1g

51521-95-0
17 suppliers
inquiry

160938-85-2
9 suppliers
inquiry

51521-95-0
17 suppliers
inquiry
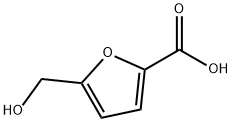
6338-41-6
191 suppliers
$5.00/100mg

51521-95-0
17 suppliers
inquiry