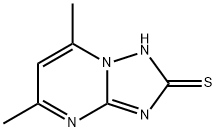
5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE synthesis
- Product Name:5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE
- CAS Number:51646-17-4
- Molecular formula:C7H8N4S
- Molecular Weight:180.23
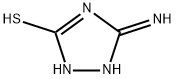
118025-51-7
0 suppliers
inquiry
123-54-6
569 suppliers
$10.00/25ml
![5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE](/CAS/GIF/51646-17-4.gif)
51646-17-4
27 suppliers
$79.75/1g
Yield:-
Reaction Conditions:
with piperidine;acetic acid at 125; for 45 h;
Steps:
1 5,7-Dimethyl-[1,2,4]-triazolo[1,5-a]pyrimidine-2-thiol
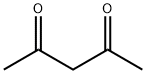
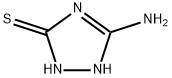
A mixture of 2,4-pentanedione (6.46 g, 64.6 mmol), 3-amino-5-mercapto-1,2,4-triazole (7.5 g, 64.6 mmol), glacial acetic acid (230 mL), and piperidine (0.58 mL, 5.9 mmol) was added to a 500 mL RBF with a magnetic stirring bar.
The flask was fitted with a condenser, and warmed in an oil bath to a gentle reflux at 125° C.
The mixture was heated for 45 hours, and solids remained visible in the flask throughout the heating period.
Progress of the reaction was monitored by analytical HPLC with UV detection at 215 and 254 nm.
The starting material 3-amino-5-mercapto-1,2,4-triazole elutes rapidly with retention time of 1.05 min, and the product elutes with retention time of 1.87 min.
The mixture was cooled to room temperature.
Solids were isolated by filtration, rinsed with 75 mL of acetic acid, and then dried under reduced pressure to afford the title compound as a pale yellow powder (9.5 g, 82% yield).
A sample of 120 mg was recrystallized in warm acetic acid to afford 60 mg (50% recovery) of fine off-white needles, while the crude powder was used in the next step. 1H NMR (DMSO-d6+CDCl3, 400 MHz) δ ppm: 14.05 (br s, 1H), 7.25 (s, 1H), 2.67 (s, 3H), 2.56 (s, 3H).
(NMR from crystalline material.)
References:
US2014/142122,2014,A1 Location in patent:Paragraph 0551; 0552

110-89-4
3 suppliers
$15.96/100ML

118025-51-7
0 suppliers
inquiry

123-54-6
569 suppliers
$10.00/25ml
![5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE](/CAS/GIF/51646-17-4.gif)
51646-17-4
27 suppliers
$79.75/1g
16691-43-3
362 suppliers
$15.00/5g

123-54-6
569 suppliers
$10.00/25ml
![5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE](/CAS/GIF/51646-17-4.gif)
51646-17-4
27 suppliers
$79.75/1g

123-54-6
569 suppliers
$10.00/25ml

16691-43-3
362 suppliers
$15.00/5g
![5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE](/CAS/GIF/51646-17-4.gif)
51646-17-4
27 suppliers
$79.75/1g
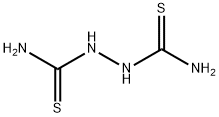
142-46-1
204 suppliers
$10.00/5g

123-54-6
569 suppliers
$10.00/25ml
![5,7-DIMETHYL[1,2,4]TRIAZOLO[1,5-A]PYRIMIDIN-2-YLHYDROSULFIDE](/CAS/GIF/51646-17-4.gif)
51646-17-4
27 suppliers
$79.75/1g