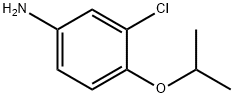
(3-chloro-4-isopropoxyphenyl)amine(SALTDATA: 0.85HCl) synthesis
- Product Name:(3-chloro-4-isopropoxyphenyl)amine(SALTDATA: 0.85HCl)
- CAS Number:5211-04-1
- Molecular formula:C9H12ClNO
- Molecular Weight:185.65
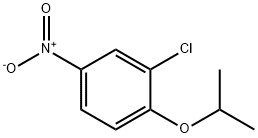
5210-99-1
4 suppliers
inquiry

5211-04-1
13 suppliers
$175.00/500mg
Yield:-
Reaction Conditions:
Stage #1: 2-Chloro-1-isopropoxy-4-nitro-benzenewith hydrogenchloride;iron in methanol at 20;
Stage #2: with sodium hydrogencarbonate in methanol;water;
Steps:
1
To a stirred mixture of 1.2 ( 1.7 g, 7.88 mmol), iron powder ( 1.3 g, 23.2 mmol) and methanol (35 mL) was added concentrated HCI (4 mL). After the reaction solution was stirred at room temperature overnight, the pH of the solution was basified by adding saturated sodium NaHCO;* and diluted with DCM (250 mL). The organic layer was washed with H?O and brine, dried over sodium sulfate, filtered, and concentrated to give 1.3, which was used without further purification in the next step.
References:
WO2007/56143,2007,A2 Location in patent:Page/Page column 51
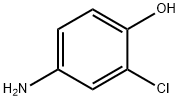
3964-52-1
250 suppliers
$20.00/5g
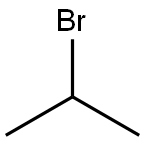
75-26-3
458 suppliers
$10.00/10g

5211-04-1
13 suppliers
$175.00/500mg