
3-ethyl 5-methyl 2-amino-1H-indole-3,5-dicarboxylate synthesis
- Product Name:3-ethyl 5-methyl 2-amino-1H-indole-3,5-dicarboxylate
- CAS Number:521286-73-7
- Molecular formula:C13H14N2O4
- Molecular Weight:262.26
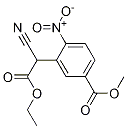
521286-72-6
7 suppliers
inquiry

521286-73-7
13 suppliers
inquiry
Yield:521286-73-7 85%
Reaction Conditions:
with acetic acid;zinc at 90 - 100; for 1 h;
Steps:
D.6 METHYL 2-AMINO-3-ETHOXYCARBONYL-1H-INDOLE-5-CARBOXYLATE
In the step D-6, ethyl a-cyano-5-methoxycarbonyl-2-nitrophenylacetate (3. 10 G) was dissolved in glacial acetic acid (30 ml) at 80°C. Keeping the temperature, zinc powder (5. 55 g) was added portionwise. The mixture was stirred at 90°C to 100°C for 1 hour. After cooling to room temperature, the reaction mixture was passed through a filter paper to remove insoluble materials and washed with glacial acetic acid (10 ml). The filtrates were combined and evaporated in vacuo. The residue was dissolved in ethyl acetate (30 ml) and washed with saturated sodium bicarbonate water solution (30 ml X 3) and brine (30 ml), successively. The organic layer was dried over sodium sulfate and evaporated in vacuo. The residue was triturated with isopropyl ether to give methyl 2-AMINO-3-ETHOXYCARBONYL-LH-INDOLE-5-CAR- boxylate as a colorless solid (2.37 g, 85%).
References:
WO2004/58764,2004,A1 Location in patent:Page 40