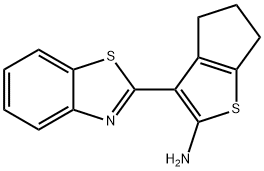
4H-Cyclopenta[b]thiophen-2-amine, 3-(2-benzothiazolyl)-5,6-dihydro- synthesis
- Product Name:4H-Cyclopenta[b]thiophen-2-amine, 3-(2-benzothiazolyl)-5,6-dihydro-
- CAS Number:522624-21-1
- Molecular formula:C14H12N2S2
- Molecular Weight:272.39
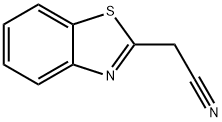
56278-50-3
161 suppliers
$15.00/1g

120-92-3
402 suppliers
$11.00/25g
![4H-Cyclopenta[b]thiophen-2-amine, 3-(2-benzothiazolyl)-5,6-dihydro-](/CAS/20210111/GIF/522624-21-1.gif)
522624-21-1
3 suppliers
inquiry
Yield:522624-21-1 69%
Reaction Conditions:
with sulfur;diethylamine in ethanol at 20;Cooling with ice;Gewald Aminoheterocycles Synthesis;
Steps:
General synthetic procedure for cycloalkylthiophene amine derivatives 4a-d
General procedure: To a solution of newly prepared 2-(benzo[d]thiazole-2-yl)acetonitrile 1a-b (5 mmol) [1], sulfur (5 mmol), cyclo-ketone (5 mmol) in alcohol (15 mL) was added diethylamine (10 mmol) dropwise under ice-bath. After that, the reaction mixture was allowed to room temperature, which was stirred and detected by TLC. Then the mixture was poured into ice water, and the formed precipitate was filtered and washed with water (20 mL) and dried in vacuo togive the yellow powder.
References:
Ke, Shaoyong;Wei, Yanhong;Yang, Ziwen;Wang, Kaimei;Liang, Ying;Shi, Liqiao [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 18,p. 5131 - 5134] Location in patent:supporting information
137-07-5
291 suppliers
$10.00/5g
![4H-Cyclopenta[b]thiophen-2-amine, 3-(2-benzothiazolyl)-5,6-dihydro-](/CAS/20210111/GIF/522624-21-1.gif)
522624-21-1
3 suppliers
inquiry