
5-(2-fluoro-phenyl)-2-Methyl-thiazole-4-carboxylic acid synthesis
- Product Name:5-(2-fluoro-phenyl)-2-Methyl-thiazole-4-carboxylic acid
- CAS Number:522646-43-1
- Molecular formula:C11H8FNO2S
- Molecular Weight:237.25
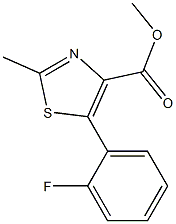
1007875-07-1
0 suppliers
inquiry

522646-43-1
18 suppliers
$175.00/100mg
Yield:522646-43-1 504 mg
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;methanol;water at 20;
Steps:
71.c
To a solution of Int. b (8.17 g, 32.52 mmol) in THF/H20/MeOH (50 mL/10 mL/50 ml_) was added UOH. H2O (6.8 g, 162.6 mmol). The mixture was stirred overnight at RT then it was concentrated under reduced pressure, a s.s. of NaHC03 was added and the mixture diluted with DCM. Phases were separated and the aqueous phase was acified with HCI 1 N until pH = 3 and extracted with AcOEt. The organic phase was washed with brine, dried over Na2S04, filtered and concentrated under vacuum. The residue was purified by FC on C18 column (eluent: from 100 % water + 0.1 % FA to 100% CH3CN + 0.1 % FA) affording 5-(2-fluorophenyl)-2-methyl-1 ,3-thiazole-4-carboxylic acid (p71 , 504 mg, y = 6.5%) as yellow solid. MS (mlz): 238.1 [MH]+.
References:
WO2019/43407,2019,A1 Location in patent:Page/Page column 96; 97

446-52-6
435 suppliers
$6.00/25g

522646-43-1
18 suppliers
$175.00/100mg
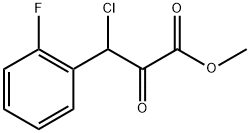
1007875-06-0
4 suppliers
inquiry

522646-43-1
18 suppliers
$175.00/100mg