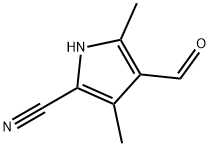
1H-Pyrrole-2-carbonitrile, 4-formyl-3,5-dimethyl- (9CI) synthesis
- Product Name:1H-Pyrrole-2-carbonitrile, 4-formyl-3,5-dimethyl- (9CI)
- CAS Number:524035-96-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
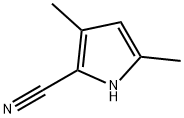
4513-92-2
8 suppliers
inquiry

524035-96-9
5 suppliers
inquiry
Yield:524035-96-9 54%
Reaction Conditions:
Stage #1: N,N-dimethyl-formamidewith trichlorophosphate for 1 h;
Stage #2: 3,5-dimethyl-1H-pyrrole-2-carbonitrile for 3.5 h;
Steps:
13.1
Example 13 (1) 5-Cyano-2,4-dimethylpyrrole-3-carboxyaldehyde Phosphorus oxychloride (10.2 ml, 110 mmol) was added dropwise to N,N-dimethylformamide (8.04 g, 110 mmol) and stirred for 1 hour. An N,N-dimethylformamide (25 ml) solution of 2-cyano-3,5-dimethylpyrrole (12.0 g, 100 mmol) reported in Synthesis, 1999, 46 was added dropwise over a period of 30 minutes. After stirring for 3 hours, the mixture was poured into iced water (approximately 500 g) and neutralized with solid sodium hydrogencarbonate. The reaction mixture was extracted with ethyl acetate, and washed with water and a saturated sodium chloride. The combined organic layer was dried with anhydrous sodium sulfate. The desiccant was filtered off, and the residue obtained by evaporation at reduced pressure was purified using medium pressure silica gel flash column chromatography (ethyl acetate:chloroform = 1:20 to 1:5) to afford 5-cyano-2,4-dimethylpyrrole-3-carboxyaldehyde (7.96 g, 54%) as a light yellow solid. Melting point: 208-210°C 1H-NMR (CDCl3): δ (ppm) 2.46 (s, 3H), 2.57 (s, 3H), 9.10 (brs, 1H), 9.97 (s, 1H).
References:
EP1911755,2008,A1 Location in patent:Page/Page column 29