
8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE synthesis
- Product Name:8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE
- CAS Number:524724-72-9
- Molecular formula:C15H12N2O
- Molecular Weight:236.27
![8-METHYL-2-PHENYLIMIDAZO[1,2-A]PYRIDINE](/CAS/20180601/GIF/885-89-2.gif)
885-89-2
20 suppliers
inquiry
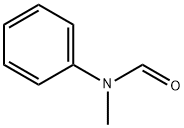
93-61-8
283 suppliers
$10.00/5G
![8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE](/StructureFile/ChemBookStructure6/GIF/CB9438569.gif)
524724-72-9
24 suppliers
$252.00/500mg
Yield:524724-72-9 98.6%
Reaction Conditions:
with tert.-butylhydroperoxide;cyclopentadienyltricarbonyliron;[copper(II)(trifluoroacetylacetonate)2];N-butylpyridine p-toluenesulfonate in chlorobenzene at 80; for 10 h;Reagent/catalyst;
Steps:
2
An appropriate amount of organic solvent chlorobenzene was added to the dry reaction vessel and then 100 mmol of the compound of formula (II), 150 mmol of the compound of formula (III), 4 mmol of copper trifluoroacetylacetonate, 220 mmol of TBHP and 7 mmol of reaction aid (6.2 mmol of N A mixture of butylpyridine p-toluenesulfonate and 0.8 mmol of cyclooctatetriene tricarbonyl iron) was added elevated temperature to 80 ° C and reacted at that temperature for 10 hours.After completion of the reaction, the mixture was allowed to cool to room temperature, and then the saturated aqueous solution of sodium bicarbonate was sufficiently washed. The organic phase was separated, dried over anhydrous magnesium sulfate and then concentrated under reduced pressure. The residue was separated by silica gel column chromatography (column chromatography The mixture was a mixture of ethyl acetate and n-propanol, and the volume ratio of the two was 10: 2) to give the compound of formula (I) in a yield of 98.6%.
References:
CN104592225,2016,B Location in patent:Paragraph 0038-0041
![8-METHYL-2-PHENYLIMIDAZO[1,2-A]PYRIDINE](/CAS/20180601/GIF/885-89-2.gif)
885-89-2
20 suppliers
inquiry
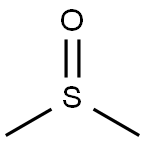
67-68-5
1214 suppliers
$15.00/100g
![8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE](/StructureFile/ChemBookStructure6/GIF/CB9438569.gif)
524724-72-9
24 suppliers
$252.00/500mg
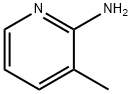
1603-40-3
479 suppliers
$6.00/25g
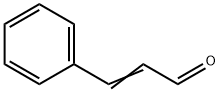
104-55-2
643 suppliers
$6.00/25g
![8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE](/StructureFile/ChemBookStructure6/GIF/CB9438569.gif)
524724-72-9
24 suppliers
$252.00/500mg
![8-METHYL-2-PHENYLIMIDAZO[1,2-A]PYRIDINE](/CAS/20180601/GIF/885-89-2.gif)
885-89-2
20 suppliers
inquiry
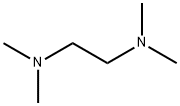
110-18-9
525 suppliers
$10.00/5mL
![8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE](/StructureFile/ChemBookStructure6/GIF/CB9438569.gif)
524724-72-9
24 suppliers
$252.00/500mg

1603-40-3
479 suppliers
$6.00/25g
![8-METHYL-2-PHENYL-IMIDAZO[1,2-A]PYRIDINE-3-CARBOXALDEHYDE](/StructureFile/ChemBookStructure6/GIF/CB9438569.gif)
524724-72-9
24 suppliers
$252.00/500mg