
4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE synthesis
- Product Name:4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE
- CAS Number:5263-78-5
- Molecular formula:C11H13NO3
- Molecular Weight:207.23
![4-METHOXY-7,8-DIHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/484-30-0.gif)
484-30-0
2 suppliers
inquiry
![4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/5263-78-5.gif)
5263-78-5
2 suppliers
inquiry
Yield:5263-78-5 67%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 20; for 18 h;
Steps:
To a solution of compound A0061-9 (2.16 g, 10.5 mmol) in methanol (45 ml) was added NaBH4 (2.0 g, 52.7 mmol) at 0 0C. The reaction mixture was stirred overnight (about 18 hours) at room temperature. TLC suggested the reaction complete. The reaction mixture was concentrated to remove the solvent and the residue was dissolved in ethyl acetate and washed with H2O and brine, dried, concentrated under vacuum to afford 2.1g of crude product. The crude product was dissolved in methanol (5 ml) and to the mixture methanol/hydrochloric acid was added until pH 1-2. The reaction mixture was concentrated to remove the solvent, diethyl ether was added to the residue and a white solid appeared. The solid was filtered and was washed with diethyl ether three times and dried to afford 1.708 g of final product (yield: 67 %) .
References:
WO2010/51374,2010,A1 Location in patent:Page/Page column 127

5779-99-7
10 suppliers
inquiry
![4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/5263-78-5.gif)
5263-78-5
2 suppliers
inquiry
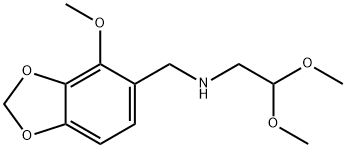
108460-85-1
9 suppliers
inquiry
![4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/5263-78-5.gif)
5263-78-5
2 suppliers
inquiry
![1,3-Dioxolo[4,5-g]isoquinolin-5(6H)-one, 7,8-dihydro-4-methoxy-](/CAS/20210305/GIF/5361-65-9.gif)
5361-65-9
0 suppliers
inquiry
![4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/5263-78-5.gif)
5263-78-5
2 suppliers
inquiry
![4-methoxy-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-8-ol](/CAS/20180629/GIF/127063-97-2.gif)
127063-97-2
1 suppliers
inquiry
![4-METHOXY-5,6,7,8-TETRAHYDRO-[1,3]DIOXOLO[4,5-G]ISOQUINOLINE](/CAS/GIF/5263-78-5.gif)
5263-78-5
2 suppliers
inquiry