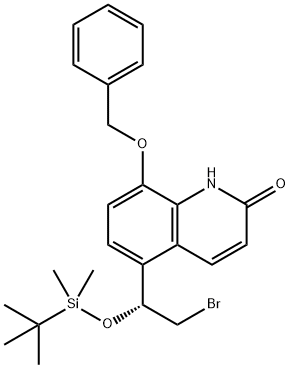
(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one ) synthesis
- Product Name:(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )
- CAS Number:530084-74-3
- Molecular formula:C24H30BrNO3Si
- Molecular Weight:488.49
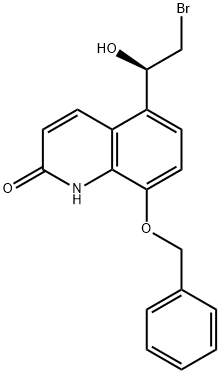
530084-79-8
138 suppliers
$16.00/100mg

18162-48-6
664 suppliers
$9.00/5g
![(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )](/CAS/20150408/GIF/530084-74-3.gif)
530084-74-3
21 suppliers
inquiry
Yield:530084-74-3 99.3%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
1 8-benzyloxy-5-((R)-2-bromo-1-(tert-butyldimethylsilyl)oxy-ethyl)-1H-quinolin-2-one synthesis (compound of formula IV)
50 g of the compound of formula III was added to a 1000 mL three-necked flask, 600 ml of dichloromethane, dissolved at room temperature, dissolved, and then added with 17 g of triethylamine, cooled to 0 ° C in an ice bath, and tert-butyldimethylchlorosilane was added dropwise with stirring. 24.8g, after adding, return to normal temperature reaction for 2~4h. After the reaction was completed, it was cooled to 0 ° C, and the solution was slowly added dropwise to a solution of IN HCl, and the layers were separated. The aqueous phase was extracted with 200 ml of dichloromethane.Concentrated to obtain 78g of the target compound,The yield is 99.3%
References:
CN110128339,2019,A Location in patent:Paragraph 0030-0032

530084-79-8
138 suppliers
$16.00/100mg
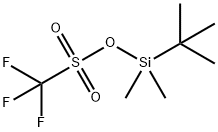
69739-34-0
361 suppliers
$10.00/1g
![(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )](/CAS/20150408/GIF/530084-74-3.gif)
530084-74-3
21 suppliers
inquiry

69739-34-0
361 suppliers
$10.00/1g
![(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )](/CAS/20150408/GIF/530084-74-3.gif)
530084-74-3
21 suppliers
inquiry
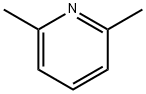
108-48-5
465 suppliers
$9.90/190μL

530084-79-8
138 suppliers
$16.00/100mg

69739-34-0
361 suppliers
$10.00/1g
![(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )](/CAS/20150408/GIF/530084-74-3.gif)
530084-74-3
21 suppliers
inquiry

108-48-5
465 suppliers
$9.90/190μL

530084-79-8
138 suppliers
$16.00/100mg
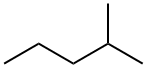
107-83-5
216 suppliers
$10.00/1g

69739-34-0
361 suppliers
$10.00/1g
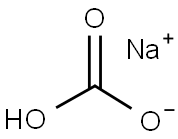
144-55-8
1304 suppliers
$10.00/25g
![(8-(benzyloxy)-5-[(1R)-2-broMo-1-{[tert-butyl(diMethyl)silyl]oxy}ethyl]quinolin-2(1H)-one )](/CAS/20150408/GIF/530084-74-3.gif)
530084-74-3
21 suppliers
inquiry