
(2S,3S,4S,5S,6S)-3,4,5-Trihydroxy-6-methoxytetrahydro-2H-pyran-2-carboxylic acid synthesis
- Product Name:(2S,3S,4S,5S,6S)-3,4,5-Trihydroxy-6-methoxytetrahydro-2H-pyran-2-carboxylic acid
- CAS Number:5391-16-2
- Molecular formula:C7H12O7
- Molecular Weight:208.17
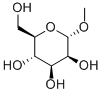
617-04-9
259 suppliers
$11.00/5g

5391-16-2
1 suppliers
inquiry
Yield:5391-16-2 90.6%
Reaction Conditions:
with calcium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical in water at 20; for 1 h;
Steps:
3
In a 50mL three-necked round bottom flask, add 0.39g (2mmol) of methyl-α-D-mannopyranoside, 0.032g (0.02mmol) of TEMPO and 30mL of water in sequence;Add 0.57g (4mmol) of calcium hypochlorite solid in batches at a temperature of 20°C,The feeding time is 10min. After the feeding is finished, the catalytic oxidation reaction is carried out.The reaction time is 1h;After the completion of the reaction, the mixture was separated by standing and filtered under reduced pressure. After adjusting the pH to 2 with 5mol/L HCl, the filtrate was concentrated under reduced pressure to remove the solvent water, and 8 mL of methanol was added to the resulting concentrate.After shaking, filter to remove inorganic salts, and the filtrate obtained is rotary evaporated to remove the solvent.That is, 0.381 g of methyl-α-D-mannanuronic acid is obtained, the yield is 90.6%, and the purity is 99%.
References:
CN113121620,2021,A Location in patent:Paragraph 0044-0050
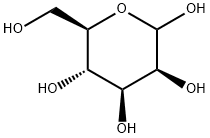
530-26-7
22 suppliers
inquiry

5391-16-2
1 suppliers
inquiry