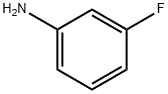
5-(3-FLUOROPHENYL)FURAN-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-(3-FLUOROPHENYL)FURAN-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:54023-07-3
- Molecular formula:C12H9FO3
- Molecular Weight:220.2
Yield:54023-07-3 87%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,2-dimethoxyethane;Inert atmosphere;Reflux;
Steps:
General procedure for the synthesis of 54a-g,i,l, 55, 56, 57
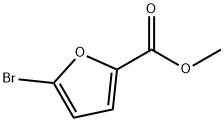
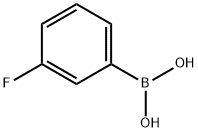
General procedure: To a stirred solution of methyl5-bromofuran-2-carboxylate 49 (1.0equiv) and Pd(PPh3)4 (0.05 equiv or 0.1 equiv forcompound 54d) in 1,2-dimethoxyethane(0.3 M) (or DMF for compound 57),the appropriate boronic acid 50a-f,i,l,51, 52, 53 (1.4 equiv) or 50g(1 equiv) was added at rt under inert atmosphere, followed by a 2 M aqueoussolution of Na2CO3 (4.2 equiv or 8.4 equiv for compound 54d). The mixture was heated underreflux for 312 h, and then the reaction was cooled down to rt and H2Owas added. The organic layer was concentrated under vacuum, and the aqueousphase was extracted with CH2Cl2. The combined organiclayers were dried over anhydrous Na2SO4, filtered andevaporated under reduced pressure. The crude residue was purified by silica gelcolumn chromatography to afford the pure cross-coupling compound.
References:
Pismataro, Maria Chiara;Horenstein, Nicole A.;Stokes, Clare;Quadri, Marta;De Amici, Marco;Papke, Roger L.;Dallanoce, Clelia [European Journal of Medicinal Chemistry,2020,vol. 205,art. no. 112669] Location in patent:supporting information
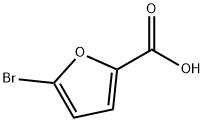
585-70-6
218 suppliers
$10.15/1g

54023-07-3
6 suppliers
inquiry