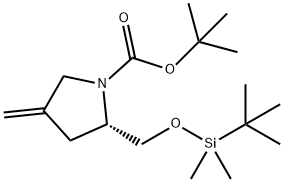
(2S)-2-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-4-methylene-1-Pyrrolidinecarboxylic acid 1,1-dimethylethyl ester synthesis
- Product Name:(2S)-2-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-4-methylene-1-Pyrrolidinecarboxylic acid 1,1-dimethylethyl ester
- CAS Number:540501-23-3
- Molecular formula:C17H33NO3Si
- Molecular Weight:327.53
1779-49-3
589 suppliers
$5.00/10g
![(2S)-2-[[tert-ButyldiMethylsilyloxy]Methyl]-4-oxo-1-pyrrolidinecarboxylic Acid tert-Butyl Ester](/CAS/GIF/220993-22-6.gif)
220993-22-6
8 suppliers
$1230.00/500mg
![(2S)-2-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-4-methylene-1-Pyrrolidinecarboxylic acid 1,1-dimethylethyl ester](/CAS/20200119/GIF/540501-23-3.gif)
540501-23-3
4 suppliers
inquiry
Yield:540501-23-3 86%
Reaction Conditions:
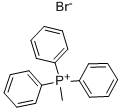
Stage #1: Methyltriphenylphosphonium bromidewith potassium tert-butylate in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Stage #2: (S)-tert-butyl 2-((tert-butyldimethylsilyloxy)methyl)-4-oxopyrrolidine-1-carboxylate in tetrahydrofuran at 0 - 20; for 4 h;Inert atmosphere;
Steps:
1 Preparation of Compound 3
Methyltriphenylphosphonium bromide (7.6 g, 21.2 mmol) was diluted with tetrahydrofuran (80 mL), and then potassium t-butoxide (1 M in THF, 21.2 mL, 21.2 mmol) was added thereto at 0°C under a nitrogen atmosphere. The mixture was stirred for 1 hour, and then a solution of Compound 2 (5.0 g, 15.2 mmol) in tetrahydrofuran (10 mL) was gradually added thereto. The mixture was stirred for 4 hours while gradually raising the reaction temperature to room temperature. A saturated aqueous ammonium chloride solution (200 mL) was added to the reaction solution and then the mixture was subjected to extraction using diethyl ether (2 × 200 mL). The combined organic layers were washed with brine (200 mL) and then dried over anhydrous sodium sulfate. The resultant was filtered, then concentrated, and purified by column chromatography to obtain Compound 3 (4.27 g, 86%). 1H-NMR (400 MHz, CDCl3) (rotamers) δ 4.97-4.91 (m, 2H), 4.09-3.93 (m, 2H), 3.84-3.80 (m, 1H), 3.65-3.61 (m, 1H), 3.59-3.34 (m, 1H), 2.64-2.55 (m, 2H), 1.69 (s, 9H), 0.87 (s, 9H), 0.03 (s, 6H).
References:
EP3604311,2020,A1 Location in patent:Paragraph 0124; 0126