4-HYDROXY-6-METHYL-3-(2-PHENYLETHANEHYDRAZONOYL)-2H-PYRAN-2-ONE synthesis
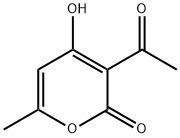
- Product Name:4-HYDROXY-6-METHYL-3-(2-PHENYLETHANEHYDRAZONOYL)-2H-PYRAN-2-ONE
- CAS Number:54107-17-4
- Molecular formula:C14H14N2O3
- Molecular Weight:258.27
Yield:54107-17-4 85%
Reaction Conditions:
in ethanol; for 1 h;Reflux;
Steps:
1-(5-Hydroxy-3-methyl-1-phenyl-pyrazol-4-yl)-3-[(4-methyl-1,3-benzothiazol-2-yl)amino]but-2-en-1-one (6)
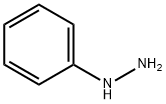
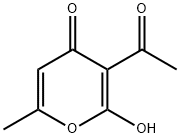
Compound 6′ was obtained by heating to reflux for 1 h a solution of DHA (1.68 g, 0.01mol) and phenylhydrazine (1.08 g, 0.01mol) in ethanol (30mL) under magnetic stirring.After cooling, the yellow precipitate was filtered, andrecrystallized in ethanol to give pure phenylhydrazone 6′(yield, 85%; mp, 200 °C). A solution of 6′ (1.29 g) washeated to reflux in acetic acid (50mL) under magnetic stirringfor 3 h. After removing the solvent under reduced pressure,the product was recrystallized in acetonitrile to afford purediketo compound 7′ as a yellow powder (yield, 73%; M.p.101 °C), which exists as two tautomeric forms, as revealed by1H-NMR (CDCl3, 300MHz) δ (ppm)=2.13 (s, CH3), 2.35(s, CH3), 2.47 (s, CH3), 3.87 (s, CH2), 5.72 (s, CH), 6.80-7.25(m, H-arom). Compound 6 was prepared by heating to refluxunder magnetic stirring a solution of 2-amino-4-methyl-1,3-benzothiazole (1.64 g, 0.01mol) and diacetoacetyl pyrazolone7′ (2.58 g, 0.01mol) in n-butanol (40mL) for 3 h. Aftercooling, the solution was then concentrated under reducedpressure to a volume of approximately 20mL, and the orangeprecipitate was filtered. Yield 70%;
References:
Moulkrere, Bachar Rébat;Orena, Beatrice S.;Mori, Giorgia;Saffon-Merceron, Nathalie;Rodriguez, Frédéric;Lherbet, Christian;Belkheiri, Nadji;Amari, Mohamed;Hoffmann, Pascal;Fodili, Mokhtar [Medicinal Chemistry Research,2018,vol. 27,# 1,p. 308 - 320]