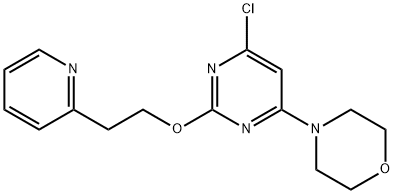
4-[6-Chloro-2-(2-(pyridin-2-yl)ethoxy)pyriMidin-4-yl]Morpholine synthesis
- Product Name:4-[6-Chloro-2-(2-(pyridin-2-yl)ethoxy)pyriMidin-4-yl]Morpholine
- CAS Number:544693-01-8
- Molecular formula:C15H17ClN4O2
- Molecular Weight:320.77
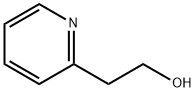
103-74-2
336 suppliers
$8.00/10g
10397-13-4
170 suppliers
$11.00/250mg
52127-83-0
68 suppliers
$95.00/1g
![4-[6-Chloro-2-(2-(pyridin-2-yl)ethoxy)pyriMidin-4-yl]Morpholine](/CAS/GIF/544693-01-8.gif)
544693-01-8
9 suppliers
$165.00/100mg
![Morpholine, 4-[2-chloro-6-[2-(2-pyridinyl)ethoxy]-4-pyrimidinyl]-](/CAS/20210305/GIF/887402-10-0.gif)
887402-10-0
0 suppliers
inquiry
![Morpholine, 4-[4-chloro-6-[2-(2-pyridinyl)ethoxy]-2-pyrimidinyl]-](/CAS/20210305/GIF/887402-09-7.gif)
887402-09-7
0 suppliers
inquiry
Yield:544693-01-8 88%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;oil at 0 - 5; for 0.5 h;
Steps:
1.2
Step 2.Product 1 (114.7g, 0.49 mol) and 2-(2-hydroxyethyl)-pyridine, 98% (64.66 g, 0.514 mol) were dissolved in anhydrous DMF (539 mL) and cooled to 0 0C. Sodium hydride, 60% dispersion in oil, (23.52 g, 0.588 mol) was added under nitrogen purge to the vigorously stirred reaction mixture in 6 portions, to maintain the reaction temperature below 5 0C. The EPO
References:
WO2006/53112,2006,A1 Location in patent:Page/Page column 51-52

103-74-2
336 suppliers
$8.00/10g

52127-83-0
68 suppliers
$95.00/1g
![4-[6-Chloro-2-(2-(pyridin-2-yl)ethoxy)pyriMidin-4-yl]Morpholine](/CAS/GIF/544693-01-8.gif)
544693-01-8
9 suppliers
$165.00/100mg