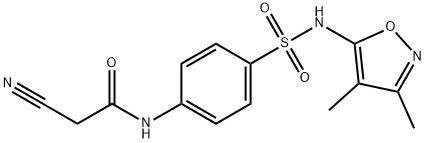
2-cyano-N-(4-{[(3,4-dimethylisoxazol-5-yl)amino]sulfonyl}phenyl)acetamide synthesis
- Product Name:2-cyano-N-(4-{[(3,4-dimethylisoxazol-5-yl)amino]sulfonyl}phenyl)acetamide
- CAS Number:546090-57-7
- Molecular formula:C14H14N4O4S
- Molecular Weight:334.35
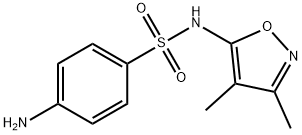
127-69-5
259 suppliers
$5.00/100mg
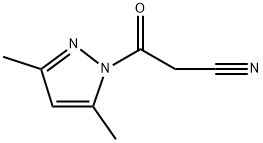
36140-83-7
91 suppliers
$23.00/250mg
![2-cyano-N-(4-{[(3,4-dimethylisoxazol-5-yl)amino]sulfonyl}phenyl)acetamide](/CAS/GIF/546090-57-7.gif)
546090-57-7
8 suppliers
inquiry
Yield:546090-57-7 82%
Reaction Conditions:
in 1,4-dioxane; for 6 h;Reflux;
Steps:
1 4.1.1. 2-Cyano-N-(4-{[(3,4-dimethylisoxazole-5-yl)amino]sulfonyl}phenyl)acetamide (3)
To a hot solution of sulfisoxazole 1 (5.34 g, 0.02 mol) in dioxan (60 mL) was added 1-cyanoacetyl-3,5-dimethylpyrazole 2 (3.26 g, 0.02 mol) and the reaction mixture was refluxed for 6 h. It was allowed to cool down to room temperature and the obtained solid was filtered off, washed with dioxan and recrystallized from ethanol/DMF to afford the target compound. Colorless crystals, yield (82%), mp 241-242 °C; IR (KBr) νmax/cm-1: 3263 (NH), 3183 (NH), 3073 (CH-Ar), 2964 (CH-sp3), 2261 (CN), 1699 (CO); 1H NMR (500 MHz, DMSO-d6): δppm = 1.63 (s, 3H, CH3), 2.09 (s, 3H, CH3), 3.98 (s, 2H, CH2), 7.72-7.77 (m, 4H, CHAr), 10.73 (s, 1H, NHSO2), 10.97 (s, 1H, NHCO); 13C NMR (125 MHz, DMSO-d6): δppm = 5.8 (CH3), 10.2 (CH3), 27.0 (CH2), 105.1 (CN), 115.5 (Phenyl-C4), 119.0 (2C, Phenyl-C2 and Phenyl-C6), 128.0 (2C, Phenyl-C3 and Phenyl-C5), 134.3 (Phenyl-C1), 142.5 (Isoxazole-C4), 155.4 (Isoxazole-C3), 161.3 (Isoxazole-C5), 161.9 (CO); MS m/z (%): 335 ([M+H]+, 22.8), 334 (M+, 24.7), 223 (100), 175 (30.2), 159 (84.8), 111 (78.8), 96 (16.2), 83 (13.7), 68 (78.2); Anal. Calcd. for C14H14N4O4S (334.35): C, 50.29; H, 4.22; N, 16.76%, Found: C, 50.33; H, 4.17; N, 16.81%.
References:
Nasr, Tamer;Bondock, Samir;Eid, Sameh [European Journal of Medicinal Chemistry,2014,vol. 84,p. 491 - 504]