
7-TRIFLUOROMETHYL-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:7-TRIFLUOROMETHYL-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:550998-55-5
- Molecular formula:C11H7F3O2S
- Molecular Weight:260.23

2365-48-2
353 suppliers
$19.00/25g
112641-20-0
141 suppliers
$10.00/1g
![7-TRIFLUOROMETHYL-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/550998-55-5.gif)
550998-55-5
21 suppliers
$86.00/500mg
Yield:550998-55-5 338 mg
Reaction Conditions:
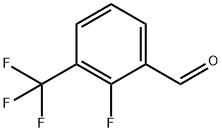
Stage #1: Methyl thioglycolate;2-fluoro-3-(trifluoromethyl)benzaldehydewith potassium carbonate in N,N-dimethyl-formamide at 140; for 2 h;
Stage #2: with oxalyl dichloride in methanol at 80; for 2 h;
Steps:
P.4 Production Example 4
A mixture of 600 mg of 2-fluoro-3-(trifluoromethyl)benzaldehyde, 398 mg of methyl thioglycolate, 694 ma of potassium carbonate and 10 ml of DMF was stirred at 140° C. for 2 hours.
The reaction mixture was cooled down to room temperature.
To the reaction mixture was added water, and the mixture was washed with tert-butyl methyl ether three times.
To the aqueous layer was added hydrochloric acid, then, the mixture was extracted with tert-butyl methyl ether three times.
The combined organic layers were washed with water and saturated saline, and dried over magnesium sulfate, then, concentrated under reduced pressure.
To the residue was added water to cause deposition of a solid which was then collected by filtration, and dried under reduced pressure.
To the resultant solid were added 20 ml of methanol and 0.31 ml of oxalyl chloride, and the mixture was stirred at 80° C. for 2 hours.
The reaction mixture was cooled down to room temperature, then, concentrated under reduced pressure.
The residue was subjected to silica gel column chromatography, to obtain 338 mg of methyl 7-(trifluoromethyl)benzo[b]thiophene-2-carboxylate (present condensed ring compound 4). [Present Condensed Ring Compound 4]. 1H-NMR (CDCl3) δ: 0.13 (s, 1H), 8.06 (m, 1H), 7.77 (m, 1H), 7.52 (m, 1H), 3.97 (s, 3H).
References:
US2014/135213,2014,A1 Location in patent:Paragraph 0108; 0109

67-56-1
756 suppliers
$9.00/25ml

2365-48-2
353 suppliers
$19.00/25g

112641-20-0
141 suppliers
$10.00/1g
![7-TRIFLUOROMETHYL-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER](/CAS/GIF/550998-55-5.gif)
550998-55-5
21 suppliers
$86.00/500mg