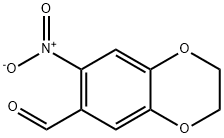
7-NITRO-2,3-DIHYDRO-1,4-BENZODIOXINE-6-CARBALDEHYDE synthesis
- Product Name:7-NITRO-2,3-DIHYDRO-1,4-BENZODIOXINE-6-CARBALDEHYDE
- CAS Number:55149-81-0
- Molecular formula:C9H7NO5
- Molecular Weight:209.16
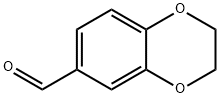
29668-44-8
269 suppliers
$6.00/1g

55149-81-0
11 suppliers
inquiry
Yield:-
Reaction Conditions:
with nitric acid at 0 - 20; for 2 h;regioselective reaction;
Steps:
2.2 Representative procedure for the synthesis of 2-aminoaldehyde (6-Aminopiperonal as example)
General procedure: Step 1 3 mL of concentrated HNO3 was added to piperonal (7) (6.66 mmol; 1g) and mixed in a round-bottom flask under ice bath. The mixture was allowed to vigorous magnetic agitate in r.t. It is observed immediate change of color (colorless to yellow). TLC reveals the end of reaction in 2h. The isolation is performed through ice-bath cooling of reactional medium, observing immediate precipitation, which was vacuum filtered, washed with around 200mL of cold distilled water and recrystallized in ethanol/water. Light yellow solid, 1.171g, 90%. Step 2 The synthesis of 6-aminopiperonalAdd 6-nitropiperonal (780 mg) to a 30 ml solution of EtOH/water (3:1) with eletrolytic iron powder (1600 mg) and concentrated HCl (1 drop). The mixture was heated at 70 °C for 1 h until the completion was detected by TLC. The reaction mixture was allowed to cool to ambient temperature. Then the reaction mass was diluted with ethyl acetate and filtered through a plug of celite. The filtrate was concentrated under reduced pressure and the resulting residue was purified by column chromatography on silica gel with ethyl acetate/petroleum ether (2:5) as eluent to afford 6-aminopiperonal(430 mg65%).
References:
Yuan, Pengtao;Gu, Xiangyu;Ni, Xintong;Qi, Yingxue;Shao, Xusheng;Xu, Xiaoyong;Liu, Jianwen;Qian, Xuhong [Bioorganic and Medicinal Chemistry Letters,2021,vol. 51,art. no. 128371] Location in patent:supporting information