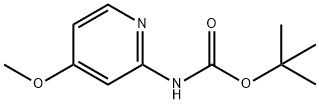
(4-METHOXY-PYRIDIN-2-YL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(4-METHOXY-PYRIDIN-2-YL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:551950-46-0
- Molecular formula:C11H16N2O3
- Molecular Weight:224.26

24424-99-5
821 suppliers
$13.50/25G
10201-73-7
263 suppliers
$8.00/1g

551950-46-0
37 suppliers
$45.00/50mg
Yield:551950-46-0 80%
Reaction Conditions:
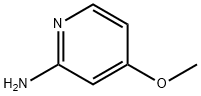
Stage #1: 2-amino-4-methoxypyridinewith lithium hexamethyldisilazane in tetrahydrofuran at 0; for 0.5 h;
Stage #2: di-tert-butyl dicarbonate in tetrahydrofuran at 20;
Steps:
5.a Tert-butyl 4-methoxypyridin-2-yl-carbamate
A mixture of 4-methoxypyridin-2-amine (1.24 g, 10 mmol) in THF (50 mL) was stirred at 0 °C, a solution of lithium hexamethyldisilazide in THF (22 mL, 22 mmol, 1.0 M) was added slowly. The resulting mixture was stirred for 30 mm. and then di-tert-butyl dicarbonate (2.26 g, 10.5 mmol) was added. The mixture was stirred at room temperature overnight. Water was added and the aqueous was extracted with EtOAc (50 mL x 3), the combined organic phase was washed with brine, dried with sodium sulfate and concentrated. The cmde product was purified by chromatography (PE:EtOAc = 30:1) to give tert-butyl 4-methoxypyridin-2- ylcarbamate (1.8 g, 80%) as a white solid. MS (ESI): m/z 225 [M+1]. ‘H NMR (400 MHz, CDC13) 9.77 (ds, 1H), 8.14 (q, J= 2.8, 5.6 Hz, 1H), 7.62 (s, 1H), 6.50 (q, J= 2.4, 6.0 Hz, 1H), 3.90 (s, 3H), 1.55 (s, 9H).
References:
WO2013/192273,2013,A1 Location in patent:Paragraph 00349

187973-18-8
39 suppliers
$90.00/50mg

551950-46-0
37 suppliers
$45.00/50mg
29681-43-4
182 suppliers
$10.00/1g

551950-46-0
37 suppliers
$45.00/50mg