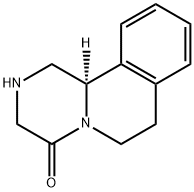
(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one synthesis
- Product Name:(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one
- CAS Number:55375-92-3
- Molecular formula:C12H14N2O
- Molecular Weight:202.25
![4H-Pyrazino[2,1-a]isoquinolin-4-one, 1,2,3,6,7,11b-hexahydro-2-[(2S)-2-(6-methoxy-2-naphthalenyl)-1-oxopropyl]-, (11bR)-](/CAS/20210305/GIF/1558003-68-1.gif)
1558003-68-1
0 suppliers
inquiry
![(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one](/CAS/20180703/GIF/55375-92-3.gif)
55375-92-3
12 suppliers
inquiry
Yield: 88%
Reaction Conditions:
with phosphoric acid in water at 150; for 0.333333 h;Microwave irradiation;Reagent/catalyst;
Steps:
(R)-(-)-1,2,3,6,7,11b-Hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one 3a
General procedure: Compound 2a (100mg, 0.241mmol) was suspended in 85w/w H3PO4 (2mL) in a proper vial which was heated to an external temperature of 150°C over a 3min period ramping up to a microwave power of 300W (Synthos 3000). The vial was held at this temperature for 20min to a microwave power of 85W. The mixture was cooled to 4°C, poured into crushed ice (15mL) and an aqueous solution of 5M NaOH was added to pH 12. The aqueous layer was extracted with dichloromethane (3×15mL). The organic layers were combined, dried over Na2SO4, filtered, and concentrated. The residue was purified by column chromatography (SiO2, dichloromethane-methanol gradient from 100:0, v/v to 90:10, v/v). A white crystalline product was obtained (43.1mg, 88% yield). mp 124-126°C. [α]D=-306.1[α]D=-306.1 (c 1, CHCl3). 1H NMR (400MHz, CDCl3) δ 7.25-7.10 (m, 4H, HC10,9,8,11), 4.86 (ddd, J=12.5, 5.1, 2.6Hz, 1H, HC6), 4.79 (dd, J=10.0, 4.6Hz, 1H, HC11b), 3.73 (ddd, J=13.0, 4.7, 1.4Hz, 1H, HC1), 3.72 (AB, J=17.6Hz, 1H, HC3), 3.52 (AB, J=17.3Hz, 1H, HC3), 3.03-2.93 (m, 1H, HC7), 2.92-2.85 (m, 1H, HC1), 2.87-2.79 (m, 1H, HC6), 2.78-2.70 (m, 1H, HC7), 1.98 (s, 1H, HN2)ppm. 13C{1H} NMR (101MHz, CDCl3) δ 167.4 (C4), 135.1 (C7a), 134.3 (C11a), 129.5 (C8), 127.1 (C9), 126.7 (C10), 124.8 (C11), 57.0 (C11b), 50.1 (C3), 49.9 (C1), 38.9 (C6), 28.9 (C7)ppm. IR νmax 3319, 2878, 1618, 1432cm-1. MS (EI) m/z (relative intensity, %) 202 (M+, 20), 173 (76), 145 (100), 131 (71), 117 (33), 103 (16), 77 (17). HRMS (EI) Calcd for C12H14N2O (M+): m/z 202.1101, Found: 202.1102. Anal. Calcd for C12H14N2O: C, 71.26; H, 6.98; N, 13.85. Found: C, 71.30; H, 6.92; N, 14.02.
References:
Cedillo-Cruz, Alberto;Aguilar, Maria Isabel;Flores-Alamo, Marcos;Palomares-Alonso, Francisca;Jung-Cook, Helgi [Tetrahedron Asymmetry,2014,vol. 25,# 2,p. 133 - 140]
61196-37-0
70 suppliers
$281.79/250mg
![(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one](/CAS/20180703/GIF/55375-92-3.gif)
55375-92-3
12 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one](/CAS/20180703/GIF/55375-92-3.gif)
55375-92-3
12 suppliers
inquiry
55268-74-1
648 suppliers
$5.00/10mg
![(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one](/CAS/20180703/GIF/55375-92-3.gif)
55375-92-3
12 suppliers
inquiry

32909-74-3
18 suppliers
$149.00/250mg
![(R)-2,3,6,7-Tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one](/CAS/20180703/GIF/55375-92-3.gif)
55375-92-3
12 suppliers
inquiry