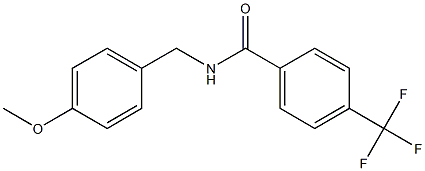
N-(4-Methoxybenzyl)-4-(trifluoroMethyl)benzaMide, 97% synthesis
- Product Name:N-(4-Methoxybenzyl)-4-(trifluoroMethyl)benzaMide, 97%
- CAS Number:554408-00-3
- Molecular formula:C16H14F3NO2
- Molecular Weight:309.2831
Yield:554408-00-3 79%
Reaction Conditions:
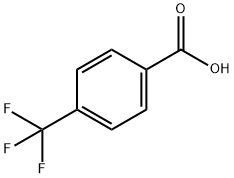
Stage #1: 4-trifluoromethylbenzoic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 0.25 h;
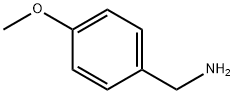
Stage #2: 4-methoxy-benzylamine in N,N-dimethyl-formamide at 20; for 5 h;
Steps:
Benzamide derivatives 25-30 using HOBt/EDC as activating agents: General method L
General procedure: A solution of 24 (1.0 equiv.), HOBt (1.1 equiv.) and EDC (1.5 equiv.) in DMF (1.3 mmol/mL) was stirred at rt for 15 minutes. The appropriate amine (2.0 equiv.) was added and the resulting solution was stirred at rt for 5 hours. Reaction media was diluted with water and aqueous phase was extracted 3 times with EtOAc. Combined organic layers were washed successively with a solution of HCl 1N, a saturated solution of NaHCO3 and brine, dried over Na2SO4 and concentrated. The resulting solid was then purified by column chromatography on silica gel using the appropriate heptanes:EtOAc mixture.
References:
Bollenbach, Maud;Lugnier, Claire;Kremer, Mélanie;Salvat, Eric;Megat, Salim;Bihel, Frédéric;Bourguignon, Jean-Jacques;Barrot, Michel;Schmitt, Martine [European Journal of Medicinal Chemistry,2019,vol. 177,p. 269 - 290] Location in patent:supporting information