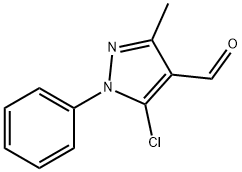
3-METHYL-5-MORPHOLIN-4-YL-1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE synthesis
- Product Name:3-METHYL-5-MORPHOLIN-4-YL-1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE
- CAS Number:5547-00-2
- Molecular formula:C15H17N3O2
- Molecular Weight:271.31
Yield:5547-00-2 85%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 90 - 100; for 8 h;
Steps:
Synthesis of 3-methyl-5-morpholino-1-phenyl-1H-pyrazole-4-carbaldehyde (6a)
General procedure: Compounds 6a-6b were prepared by heating the appropriate compound 2 (0.200 g 1 mol) with morpholine/piperidine (72.44 mml, 1.5 mol) in DMF at 90 °C in the presence of anhydrous potassium carbonate for 8 h. This reaction is proposed to proceed via SN2 mechanism. The reaction mixture was poured on ice and the precipitate formed was filtered and dried. The residue was purified on column chromatography (silica gel with 20% ethyl acetate in hexane) to afford pure compound. Yield 0.160 g, (65%); M.P. 158 °C; 1H NMR (500 MHz,) δ 2.45 (s, 3H), 3.12-3.14 (t, 4H), 3.65-3.67 (t, 4H), 7.37 (d, J = 6.6 Hz, 1H), 7.34-7.49 (m, 4H), 9.95 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 13.78,29.85, 51.03, 66.94, 76.94, 77.94, 77.45, 112.26, 125.14, 128.59, 129.45, 139.23, 151.93, 152.48, 183.41; HRMS (ESI) calcd for C15H17N3O2 [M-H]-: m/z = 271.13, found 272.1395; purity 100%.
References:
Nidhar, Manisha;Sonker, Priyanka;Sharma, Vishal Prasad;Kumar, Sanjay;Tewari, Ashish Kumar [Chemical Papers,2022,vol. 76,# 3,p. 1707 - 1720]

89-25-8
775 suppliers
$6.00/25g

5547-00-2
5 suppliers
inquiry
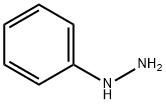
100-63-0
357 suppliers
$10.00/1g

5547-00-2
5 suppliers
inquiry