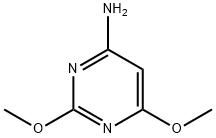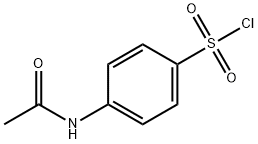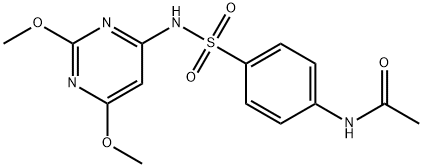
4-(Acetylamino)-N-(2,6-dimethoxy-4-pyrimidinyl)benzenesulfonamide synthesis
- Product Name:4-(Acetylamino)-N-(2,6-dimethoxy-4-pyrimidinyl)benzenesulfonamide
- CAS Number:555-25-9
- Molecular formula:C14H16N4O5S
- Molecular Weight:352.37
Yield:555-25-9 99%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 15; for 14 h;Large scale;Temperature;
Steps:
1.1 Example 1
(1) In a 2000 L dry reactor, 4-amino-2,6-dimethoxypyrimidine (456 kg)1200 kg of N, N-dimethylformamide (DMF), 303 kg of triethylamine,In stirring under the inputAcetaminobenzene sulfonyl chloride (ASC) 701kg,Internal temperature control at 15 , 2h plus finished. After adding, insulation 12h.After the tracing reaction is complete, DMF is recovered in high vacuum and excess triethylamine (applied).The autoclave was added 1200kg of ethyl acetate, heated to 65 ° C, filtered while hot,The filter cake is triethylamine hydrochloride. The filtrate was cooled to 0 ° C and centrifuged to obtain a wet product 1;Centrifuged mother liquor was concentrated to 1/4 of the volume, then cooled to 0 ° C and centrifuged to obtain wet product 2.The mixed wet product 1 and the wet product 2 are dried to obtain a condensation product4-p-acetamidobenzenesulfonamido-2,6-dimethoxypyrimidine 1024.59kg, yield 99%Content of more than 98.5%.Ethyl acetate applied, triethylamine hydrochloride for co-processing.
References:
CN107325057,2017,A Location in patent:Paragraph 0023-0026; 0032-0034; 0038; 0039; 0043; 0044