
TERT-BUTYL BIS(2-(2,2,2-TRIFLUOROACETAMIDO)ETHYL)CARBAMATE synthesis
- Product Name:TERT-BUTYL BIS(2-(2,2,2-TRIFLUOROACETAMIDO)ETHYL)CARBAMATE
- CAS Number:556082-00-9
- Molecular formula:C13H19F6N3O4
- Molecular Weight:395.3
Yield:556082-00-9 100%
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;2,2,2-trifluoro-N-[2-({2-[(trifluoroacetyl)amino]ethyl}amino)ethyl]acetamide in tetrahydrofuran at 0 - 20; for 20 h;
Stage #2: with ammonium chloride in tetrahydrofuran;water at 20; for 5 h;
Steps:
M Example M; N-[2-({2-[(1,4-Dioxido-1, 2, 4-benzotriazin-3-yl) amino] ethyl} amino) ethyl]-4- [ACRIDINECARBOXAMIDE] (37); [VERT-BUTYL] bis- (2-aminoethyl) carbamate (33).
[DIETHYLENETRIAMINE] (9.9 mL, 96 mmol) was added to a solution [OF CF3CO2ET] (22.8 [ML,] 192 mmol) in dry ether (80 mL) at [5 °C] and the reaction mixture was stirred at [20 °C] for 20 h. The resulting white precipitate was filtered and washed with cold ether (100 mL), dried under vacuum to give 2, 2, 2-trifluoro-N-[2-({2-[(trifluoroacetyl) amino] ethyl} amino) ethyl] acetamide (17.26 g, [61%), 1H NMR [(CD3) 2SO] 6] 7.26 (br, 2 H, 2 x CONH), 3.43 (br s, 4 H, 2 x CH2), 2.86 (t, [J=] 5. [8] Hz, 4 H, 2 x [CHO) ; 13C] NMR [(CD3)2SO] 8 157. 7 (q, [J=] [37] Hz), 115.8 (q, [J=] 288 Hz), 47.3 (2), 39.3 (2). [DI-TERT] butyldicarbonate (8. 26 g, 37.8 mmol) was added to a solution of acetamide (10.15 g, 34.4 mmol) in THF (100 [ML)] at [0 °C] and the mixture was stirred at 20 [°C] for 20 h. Saturated aqueous [NH4CI] [(80] [ML)] added and the mixture stirred at [20 °C FOR] 5 h. The mixture was extracted with DCM (3 x 50 mL), dried, and the solvent evaporated to give [TERT-BUTYL] bis {2-[(trifluoroacetyl) amino] ethyl} carbamate (13.5 g, 100 [%), 1H NMR [(CD3)2SO] No. ] 9.47 (br, 1 H, [CONH),] 9.40 (br, 1 H, CONH), 3. [30] [(M,] 8 H, 4 x [CH2),] 1. [38] [s, 9H, C [(CH3)] [3] ; C NMR [ (CD3) 2SO] 8] 156.4 (q, J= 36 Hz), 154.7, 115.8 (q, J = 288 Hz), 78.9, 45.4, 45.0, 37.7, 37.4, 27.7 (3). [CONC.] ammonia [(50] mL) was added to a solution of carbamate (14.0 g, [35.] 5 mmol) in MeOH (100 [ML)] and heated at reflux temperature for 20 hr. The solvent was evaporated to give diamine 33 as a yellow [FOAM, 1H NMR [(CD3)2SO] No. ]3. 38 (t, J = 6. [4 HZ, 4 H, 2 X CH2),] 2.94 (t, [J = 6.4 HZ, 4 H, 2 No. CH2), ] 1.42 [s, 9 H, C (CH3) [31 ; 13C] NMR [[(CD3) 2SO] 6] 154.9, 79. 9, 45.1 (2), [37.] 4 (2), 27.9 (3).
References:
WO2004/26846,2004,A1 Location in patent:Page 71; 72
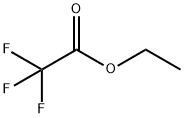
383-63-1
473 suppliers
$10.00/25g

24424-99-5
825 suppliers
$13.50/25G
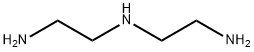
111-40-0
491 suppliers
$13.44/25ML

556082-00-9
9 suppliers
inquiry
![2,2,2-trifluoro-N-[2-({2-[(trifluoroacetyl)amino]ethyl}amino)ethyl]acetamide](/CAS/20210305/GIF/88793-42-4.gif)