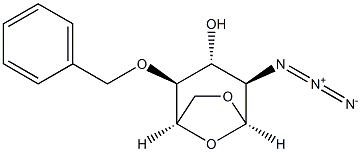
1,6-Anhydro-2-azido-2-deoxy-3,4-bis-O-(phenylmethyl)-beta-D-glucopyranose synthesis
- Product Name:1,6-Anhydro-2-azido-2-deoxy-3,4-bis-O-(phenylmethyl)-beta-D-glucopyranose
- CAS Number:55682-48-9
- Molecular formula:C20H21N3O4
- Molecular Weight:367.403
Yield:55682-48-9 506 mg
Reaction Conditions:
with pyridine
Steps:
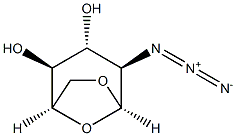
1,6-Anhydro-2-azido-3-O-benzoyl-2-deoxy-β-D-glucopyranose (12)
General procedure: Benzoyl chloride (350 μL, 3.02 mmol) was added to a solution of azide 11 [1] (415 mg, 1.50mmol) in pyridine (7 mL) and the resulting solution was stirred overnight. It was then poured onto iceand extracted with dichloromethane (3×). The extracts were combined, dried and concentrated toafford crude 3-O-benzoate (506 mg). The benzoate was dissolved in ethyl acetate (20 mL) and asolution of sodium bromate (681 mg, 4.51 mmol) in water (15 mL) was added. The resulting mixturewas stirred vigourously and a solution of sodium dithionite (784 mg, 4.51 mmol) was added dropwiseand stirring continued for 2.5 h. Sodium thiosulfate was then added to destroy excess of bromine, theorganic layer was separated and the water layer was extracted with ethyl acetate (3×). The combinedorganic phases were dried and concentrated, the residue dissolved in chloroform, the solution was washed with saturated NaHCO3 and water, dried and concentrated. Chromatography in S12 followedby recrystallization from hot ethyl acetate/heptane gave benzoate 12 (203 mg, 47%); mp 149-150 °C;[α]20D +33 (CHCl3, 0.29); (ref [2] gives 143-145 °C; [α] D20 +24 (c 0.9, CH2Cl2)); 1H NMR (500 MHz,CDCl3) agrees with that reported [2], additional coupling constants 3J2,1 = 1.5 Hz, 3J2,3 = 1.6 Hz,2J6ex,6en = 7.7 Hz, 3J6ex,5 = 5.8 Hz, 2J6en,6ex = 7.7 Hz, 3J5,6ex = 5.8 Hz, 3J3,2 = 1.6 Hz, 3J3,4 = 1.6 Hz, 4J3,1 =1.6 Hz, 4J3,5 = 1.6 Hz, 3J1,2 = 1.5 Hz, 4J1,3 = 1.6 Hz; 13C {1H} NMR (125 MHz, CDCl3) δ 59.4 (C-2),65.2 (C-6), 68.6 (C-4), 72.2 (C-3), 76.1 (C-5), 100.0 (C-1), 128.7 (CH, Bz), 129.1 (Cipso), 129.7, 133.7(2 × CH, Bz), 165.3 (CO, Bz). HRMS (ESI) m/z [M + Na]+ calcd for C13H13N3NaO5: 314.0747; found:314.0752.
References:
Horník, ?těpán;?t'astná, Lucie ?ervenková;Cu?ínová, Petra;Sykora, Jan;Káňová, Kate?ina;Hrstka, Roman;Císa?ová, Ivana;Dra?ínsky, Martin;Karban, Jind?ich [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 750 - 759] Location in patent:supporting information
67227-89-8
0 suppliers
inquiry

55682-48-9
35 suppliers
inquiry