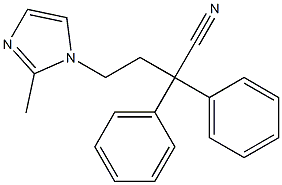
IMidafenacin IMpurity (1-(3-Cyano-3,3-Diphenylpropyl)-2-Methyl-1H-IMidazoliuM Phosphate) synthesis
- Product Name:IMidafenacin IMpurity (1-(3-Cyano-3,3-Diphenylpropyl)-2-Methyl-1H-IMidazoliuM Phosphate)
- CAS Number:562091-56-9
- Molecular formula:C20H22N3O4P
- Molecular Weight:399.3801
214777-43-2
18 suppliers
inquiry

562091-56-9
9 suppliers
inquiry
Yield:562091-56-9 74.5%
Reaction Conditions:
with phosphoric acid in ethanol;water at 30 - 31; for 16.5 h;
Steps:
1
Referential example 1; Crude crystals of 4-(2-methylimidazole-1-yl)-2,2-diphenylbutanamide A mixture of 500g (1.67mol) of 4-bromo-2,2-diphenylbutyronitrile , 685g (8.34mol) of 2-methylimidazole and 250mL of dimethylsulfoxide (DMSO) was stirred for 5 hours at 95 to 105°C, and then cooled with ice water. After 2L of ethyl acetate and 2L of water were added therein at an inner temperature of 39°C, and the mixture was stirred for 5 minutes, organic layer was separated. After washing the organic layer with 2L- of water and then 2L of 2.5% acetic acid, the organic layer was concentrated under reduced pressure. The residual oil was dissolved in 2L of ethanol and to this solution a solution of 192g (1.67mol) of 85% phosphoric acid and 1L of ethanol was added dropwise at an inner temperature of 31°C under stirring, and this dropwise addition was interrupted when the solution became milky white (about 500mL were added dropwise). After confirming the precipitation of crystals by stirring for 30 minutes, remaining phosphoric acid solutionwas added dropwise, and successively the solutionwas stirred for 16 hours at an inner temperature of 30°C. The precipitated crystals were collected by filtration and, afterwashing them with 1L of ethanol, the crystals were dried for 5 hours at 60°C under reduced pressure (vacuum pump) to obtain 496g (74.5%) of 4-(2-methylimidazole -1-yl)-2,2-diphenylbutyronitrile phosphate. Out of these crystals, 100g were completely dissolved with stirring into a mixture of 100mL of purified water and 400mL of 2-propanol and to this solution 500mL of 2-propanol were added under stirring, followed by cooling with ice water. After stirring this solution for 1 hour at an inner temperature of below 15°C, the precipitated crystals were collected by filtration which were then washed with 100mL of 2-propanol, then with 100mL of ethyl acetate. The crystals were dried for 17 hours at 60°C under reduced pressure (vacuum pump) to obtain 91.3g (Total yield: 68%) of purified product of 4-(2-methylimidazole-1-yl) -2,2-diphenylbutyronitrile phosphate as white crystalline powder with hygroscopicity . After a mixture of 80.0g (200mmol) of the purified product thus obtained, 132g (2.02mol) of 86% potassium hydroxide and 400mL of 2-propanol was refluxed for 5 hours under an atmosphere of argon, the mixture was cooled with ice water. Under stirring, 800mL of 2mol/L hydrochloric acid were added into the mixture at an inner temperature of 30°C (temperature was raised to 50°C) to crystallize out of the mixture, and, after stirring for 1 hour at an inner temperature of below 15°C, the crystals were collected from the solution by filtration. These crystals were washed with a mixture of 30mL of 2-propanol and 60mL of purified water, then with each 250mL of purified water five times (fifth washed solution: pH 8.80). The crystals were dried for 16 hours at 40°C in blower to obtain 55.2g (86.4%) of crude crystals. Into a mixed solution of 200mL of 95% 2-propanol, 27.6g out of these were dissolved completely by heating, which was then stirred for 1 hour at room temperature to crystallize out of the solution, followed by cooling with ice water. After stirring the solution for 1 hour at an inner temperature of below 15°C, the precipitated crystals were collected by filtration and washed with 10mL of 2-propanol. The crystals were dried for 3hours at 60°C under reduced pressure (vacuum pump) to obtain 25.7g(Total yield: 80%) of crude crystals of 4-(2-methylimidazole-1-yl)-2,2-diphenylbutanamide as white crystalline powder.
References:
EP1845091,2007,A1 Location in patent:Page/Page column 4