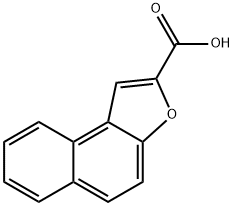
naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE) synthesis
- Product Name:naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)
- CAS Number:5656-67-7
- Molecular formula:C13H8O3
- Molecular Weight:212.2

105-36-2
354 suppliers
$5.00/5G
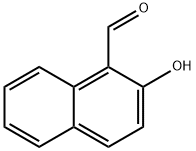
708-06-5
297 suppliers
$14.00/5g
![naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)](/CAS/GIF/5656-67-7.gif)
5656-67-7
16 suppliers
$133.00/5G
![ETHYL NAPHTHO[2,1-B]FURAN-2-CARBOXYLATE](/StructureFile/ChemBookStructure5/GIF/CB8243638.gif)
32730-03-3
16 suppliers
$362.00/500mg
Yield:5656-67-7 92% ,32730-03-3 5%
Reaction Conditions:
Stage #1: 2-hydroxynaphthalene-1-carbaldehydewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.5 h;Inert atmosphere;
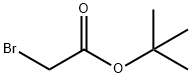
Stage #2: ethyl bromoacetate in N,N-dimethyl-formamide at 140; for 22 h;
Steps:
1 Example 1 Synthesis of naphtho[2,1-b]furan-2-carboxylic acid (2a)
Under a nitrogen atmosphere, anhydrous potassium carbonate (K2CO3) (64.13 g, 290 mmol) was added to a solution of 2-hydroxy-1-naphthaldehyde (1) (10 g, 58 mmol) in anhydrous dimethylformamide (DMF) (150 mL). The mixture was stirred at room temperature for 30 minutes, to which ethyl bromoacetate (8.36 mL, 75 mmol) was added. Then, the resulting reaction mixture was heated at 140° C., stirred for 22 hours, and cooled at room temperature, and the reaction mixture was poured to ice cold water. The resulting solid was filtered, washed with water, and dried. Then, the product was recrystallized from diethyl ether to yield a purified product of naphtho[2,1-b]furan-2-carboxylic acid (2a) as a pale brown solid (11.3 g, yield 92%) (Rf=0.14) and ethyl naphtho[2,1-b]furan-2-carboxylic acid (2b) as a yellow solid (0.7 g, yield 5%) (Rf=0.87) (hexane:ethyl acetate=1:1). The 1H NMR and 13C NMR spectra of the resulting naphtho[2,1-b]furan-2-carboxylic acid (2a) is shown in FIG. 1, and the mass spectrum is shown in FIG. 2. 1H NMR (400 MHz, DMSO-d6) δ ppm: 8.269-8.250 (1H, d, J=7.6, Ar-H), 8.000-7.980 (1H, d, J=8, Ar-H), 7.788-7.765 (1H, d, J=9.2, Ar-H), 7.727-7.704 (1H, d, J=9.2, Ar-H), 7.558-7.599 (1H, t, J=7.6, Ar-H), 7.541 (1H, s, Ar-H), 7.498-7.460 (1H, t, J=7.6, Ar-H); 13C NMR (100 MHz, DMSO-d6); δppm: 161.293, 156.558, 150.965, 129.704, 128.491, 127.679, 126.142, 124.875, 124.263, 123.6, 123.465, 112.824, 105.238; ESIMS found: m/z 211.4[M-H]-.
References:
US2016/102078,2016,A1 Location in patent:Paragraph 0060-0063
5292-43-3
452 suppliers
$10.00/5g

708-06-5
297 suppliers
$14.00/5g
![naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)](/CAS/GIF/5656-67-7.gif)
5656-67-7
16 suppliers
$133.00/5G

708-06-5
297 suppliers
$14.00/5g
![naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)](/CAS/GIF/5656-67-7.gif)
5656-67-7
16 suppliers
$133.00/5G
![ETHYL 2-[(1-FORMYL-2-NAPHTHYL)OXY]ACETATE](/CAS/GIF/76322-09-3.gif)
76322-09-3
23 suppliers
$118.00/500mg
![naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)](/CAS/GIF/5656-67-7.gif)
5656-67-7
16 suppliers
$133.00/5G
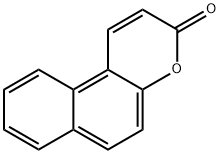
4352-89-0
24 suppliers
inquiry
![naphtho[2,1-b]furan-2-carboxylic acid(SALTDATA: FREE)](/CAS/GIF/5656-67-7.gif)
5656-67-7
16 suppliers
$133.00/5G